应用透射电镜技术研究栽培甜菜(
An experiment was conducted by using TEM to study the degenerative process of synergid in sugar beet, so as to provide more information for reproductive biology of angiosperm. The results were as follows: two synergids were similar in flower bud stage with large number of organelles. Both of them had developed filiform apparatus (FA) at micropyle end and lacked cell wall at chalazal end, showing obvious polarity. Then electron density in one synergid increased. On the other hand, vacuole membrane disappeared, membrane of mitochondrium, plastid and nuclear became illegible, which suggested cell degeneration began. But endoplasmic reticulum and Golgi bodies were still active. Complete degeneration of this synergid occurred before pollination. Number of organelles including mitochondrium, plastids and ribosomes gradually increased in the other synergid (persistent synergid). Mitochondria contained many tubular cristae and obvious DNA fibril. Plastids were irregular in shape and usually contained starch gains and thylakoid membranes. The metabolism of persistent synergid gradually enhanced until fertilization (about 13 h after pollination). It began to degenerate when zygote had alveolate cell wall at the chalazal end and endosperm occurred, while complete degeneration and disappearance of FA took place at late stage of zygote. The results indicated that degeneration of one synergid in sugar beet must be triggered by other stimulation than pollination and pollen tube growth. As a transfer cell, persistent synergid may absorb and transport nutrition for the development of embryo sac.
被子植物受精作用是近年在植物生殖生物学中最受关注的课题之一, 受精过程包括花粉在柱头上萌发和长出花粉管; 花粉管生长通过花柱, 进入胚囊和释放内容物; 配子融合[ 1]。助细胞退化是受精过程能否顺利完成的不可或缺的环节, 具有重要功能[ 2]。目前已在大量材料的超微结构研究中报道了助细胞退化[ 3, 4, 5, 6, 7, 8, 9, 10], 并对助细胞退化时间和功能进行了总结[ 1, 11]。然而对助细胞退化进程的描述较少[ 3, 12, 13]。关于助细胞退化机制虽有较多研究[ 14, 15], 但尚薄弱, 仍没有明确结论。栽培甜菜为我国重要的糖料作物, 目前对其大小孢子发生、雌雄配子体发育[ 16, 17, 18]、花粉[ 19, 20]、成熟胚囊[ 5]、卵细胞、合子及二细胞原胚[ 21]均进行了电镜水平研究, 并在光镜水平对其受精作用[ 22]进行了研究。本研究详尽描述栽培甜菜助细胞退化进程的超微结构特征, 探讨了助细胞退化时间及机制, 以进一步完善甜菜生殖生物学研究及丰富被子植物生殖生物学基础资料。
将栽培甜菜双丰1号( Beta vulgaris)种植于黑龙江大学植物园。2004—2006年, 每年6月末至7月中旬, 取直径为1.5~3.0 mm的花蕾及刚开放的小花, 剥离胚珠并迅速置3%戊二醛(pH 7.2, 二甲胂酸钠缓冲液配制)固定; 每天清晨8:00对刚开的花朵进行人工异花授粉, 授粉后13~27 h内, 每2 h取雌蕊50个, 剥离胚珠并用3%戊二醛固定。以缓冲液冲洗4次, 再用1%锇酸室温下固定2~4 h, 缓冲液冲洗后, 系列乙醇脱水, 经环氧丙烷置换, Spurr树脂渗透包埋。用Leica Ultracut R型超薄切片机切片(厚度70 nm), 经醋酸双氧铀-柠檬酸铅双重染色后, 在JEOL-1230型透射电镜下观察并拍照。
栽培甜菜雌配子体发育类型为蓼型。2个助细胞位于胚囊的珠孔端, 其合点端与中央细胞相邻。卵细胞在侧面与2个助细胞依附, 横切面上, 卵细胞与两助细胞呈“品”字形排列。2个助细胞分别在受精前、后退化, 其退化经历如下几个阶段。
花蕾时期, 2个助细胞同时存在且已发育成熟(图版I-A)。助细胞高度特化, 具独特的细胞壁结构。助细胞珠孔端的壁与胚囊壁结合, 液泡化的发展使助细胞向中央细胞方向扩展, 进而在靠近珠孔端形成“钩”状结构。助细胞珠孔端壁增厚, 向胞质形成众多指状突起——丝状器。助细胞的细胞壁由珠孔端向合点端逐渐变薄, 在合点端以质膜形式与中央细胞接触(图版I-A)。助细胞的极性明显, 液泡位于合点端占据细胞一半的体积, 细胞核紧靠液泡位于细胞的中部, 细胞质集中在核与丝状器之间(图版I-A)。2个助细胞的核大小相似, 胞质相似, 线粒体堆叠在丝状器旁, 但内嵴不明显, 电子较透明; 质体较少且电子不透明(图版I-B); 粗面内质网数目众多, 呈囊泡状, 分布于核周围; 高尔基体大量存在, 并活跃地分泌小泡(图版I-C, D)。超微结构特征显示助细胞是代谢活跃的细胞。
花蕾期随着发育的进行, 2个助细胞开始出现差异, 一个助细胞的细胞核与细胞质染色加深, 电子密度增高, 呈现退化迹象(图版I-E)。此助细胞的大液泡消失, 液泡膜不可见, 细胞体积变小。线粒体内嵴明显, 但有的线粒体膜结构开始不清晰(图版I-F); 粗面内质网大量汇集在细胞的合点端, 潴泡彼此平行排列, 末端膨大(图版II-A); 高尔基体微弯曲, 仍分泌小泡(图版II-B)。
另一个助细胞宿存, 染色较浅, 超微结构没有明显改变(图版I-E)。只是线粒体数量增加, 且内嵴发达, DNA纤丝清晰可见(图版II-D); 质体中出现淀粉粒(图版II-C); 大量核糖体附着在内质网上。虽然两助细胞出现差异, 但两细胞之间的细胞壁上仍有胞间连丝联系(图版I-F)。
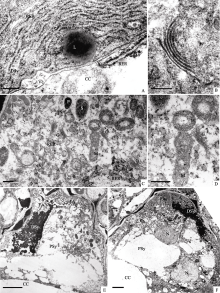 | 图版II (A~D): 图版I中图E的局部放大, 图A, B示开始退化助细胞的胞质(Bar = 200 nm); 图C, D示宿存助细胞中的胞质(Bar = 500 nm)。(E, F): 一个助细胞完全退化, 一个助细胞宿存, 箭头示卵细胞合点端的电子浓密物质(图E, Bar = 5 μm; 图F, Bar = 2 μm)。Plate II (A-D): Higher magnification of Figure E in Plate I, Figure A and B show cytoplasm of the degenerated synergid; Figure C and D show cytoplasm of the persistant synergid (Bar = 500 nm). (E, F): One synergid degenerated completely, the other persisted, electronic dense material at chalazal end of egg cell shown by arrows (Figure E, Bar = 5 μm; Figure F, Bar = 2 μm). |
花蕾刚开放时(授粉前), 一个助细胞完全退化, 仅剩下退化残迹, 丝状器仍然存在, 但无法分辨细胞器结构(图版II-E)。电子浓密物质出现在卵细胞与中央细胞之间的膜间隙(图版II-F)。而宿存助细胞内的细胞器明显增多, 代谢加强(图版II-F)。表现为线粒体、质体、内质网、高尔基体及核糖体数量增多, 胞质电子密度增加。线粒体具发达内嵴; 管状或囊泡状的粗面内质网潴泡相互联结, 其上结合众多核糖体; 高尔基体活跃地分泌小泡(图版III-A, B)。
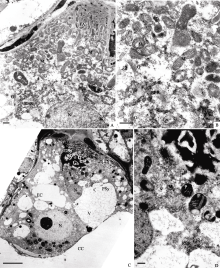 | 图版III (A, B): 图版II中图F的局部放大, 示宿存助细胞的胞质(Bar = 500 nm)。(C): 受精前的宿存助细胞(Bar = 5 μm)。 (D): 图C的局部放大, 示宿存助细胞的胞质(Bar = 500 nm)。Plate III (A, B): Higher magnification of Figure F in Plate II, showing cytoplasm of the persistant synergid (Bar = 500 nm). (C): Persistant synergid before fertilization (Bar = 5 μm). (D): Higher magnification of Figure C, showing cytoplasm in persistant synergid (Bar = 500 nm). |
授粉后约13 h, 卵细胞成熟, 呈洋梨形, 且与中央细胞间的膜间隙增大(图版III-C)。宿存助细胞中质体数量进一步增加, 且形态多样, 有的弯曲, 有的呈杯状或球形, 可见质体的片层(图版III-D)。有脂体出现; 胞质富含核糖体, 电子密度加深(图版IV-A); 其他细胞器没有明显变化。可见, 宿存助细胞的代谢达到最活跃状态。
授粉后约21 h, 合子的合点端已形成蜂窝状的细胞壁(图版IV-B), 初生胚乳核已分裂为游离核(另文报道), 一侧的退化助细胞中仍可见丝状器, 另一侧的宿存助细胞染色加深, 电子密度增高, 其内的细胞器膜模糊不清, 开始出现退化迹象(图版IV-B, C)。授粉后约25 h, 合子的合点端已形成完整的细胞壁, 宿存助细胞完全退化, 丝状器消失(图版IV-D)。
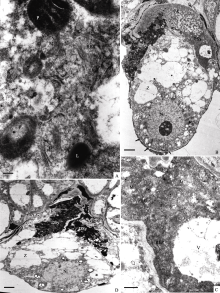 | 图版IV (A): 图版III中图C的局部放大, 示宿存助细胞的胞质(Bar = 200 nm)。(B): 合子时期的助细胞, 箭头示合子合点端蜂窝状细胞壁(Bar = 2 μm)。(C): 图B的局部放大, 示宿存助细胞的胞质(Bar = 500 nm)。(D): 合子后期, 2个助细胞均退化(Bar = 2 μm)。Plate IV (A): Higher magnification of Figure C in plate III, showing cytoplasm of the persistant synergid (Bar = 200 nm). (B): Synergids at zygote stage, arrow shows cell wall at chalazal end of zygote (Bar = 2 μm). (C): Higher magnification of Figure B, showing persistant synergid began to degenerate (Bar = 500 nm). (D): Both of two synergids degenerated at late stage of zygote (Bar = 2 μm). |
20世纪60年代以来, 大量超微结构的观察, 得出一致的结论, 即除缺少助细胞的白花丹属植物外, 花粉管都是到达胚囊中的一个助细胞[ 1]。接受花粉管的助细胞或迟或早退化, 其退化的时间在不同植物中存在差异, 有的助细胞在授粉前开始退化, 如小麦[ 3]、水稻[ 4]和甜菜[ 5]; 有的助细胞的退化发生在授粉后至花粉管到达胚囊前这一段时间, 大多数植物属于此种类型, 如栎[ 6]、大豆[ 7]、陆地棉[ 8]等; 有的助细胞在花粉管进入后不久退化, 如荠菜[ 9]。其中, 在小麦中描述了助细胞的退化进程, 即花蕾期2个助细胞均有退化迹象, 花粉管到达之前不再继续退化, 而花粉管进入助细胞并释放内容物导致此助细胞进一步退化。本研究发现甜菜的一个助细胞在花蕾时期开始出现退化迹象, 至花蕾开放(授粉前)即完全退化, 仅留下退化残迹。
助细胞并不是简单意义的退化, 而是受精过程中的一个关键性程序, 具有重要的生理功能[ 2]。大量工作暗示助细胞可能具有吸引花粉管的作用, 离体实验证明花粉管的生长是直接或间接由助细胞的某种成分或其释放的某种物质所引导的, 但是这种起源于助细胞的信号尚未确定[ 23]。Jensen[ 10]首先在陆地棉中用显微灰化法发现助细胞富含灰分, 推测其中存在大量钙, 进一步提出假设, 假定助细胞退化时, 随着液泡膜的破坏, 储存在液泡内高浓度的钙盐被释放出来, 形成助细胞与相邻珠心细胞的钙的梯度分布, 进而引导花粉管向助细胞生长并进入其中。花粉管在退化助细胞的高钙环境中停止生长并释放内容物[ 14]。本研究中栽培甜菜花蕾期助细胞开始退化时, 液泡膜消失, 质体和线粒体膜以及核膜不清晰, 而内质网异常发达, 高尔基体分泌小泡, 显示分泌活动活跃; 另外, 此助细胞退化后, 其丝状器仍存在。因而我们推测退化助细胞通过丝状器分泌某种物质(所分泌物质有待进一步检测), 引导花粉管的定向生长, 使之准确到达退化助细胞中并释放内容物。花蕾刚开放时, 退化助细胞完全退化, 细胞内的电子浓密物质转移至卵细胞与中央细胞之间的膜间隙, 这与Brunn[ 5]的描述相同, 推测与精细胞转移至受精靶区相关。
宿存助细胞的宿存时间长短因植物而异, 有的宿存时间较长, 并维持活跃的生理状态, 如向日葵[ 24]的助细胞在心形胚时期仍存在。本研究中, 栽培甜菜的一个助细胞退化后, 另一个助细胞(宿存助细胞)的细胞器逐渐增多, 功能状态趋于活跃, 表现在核糖体、线粒体、质体等数量增加; 线粒体内嵴丰富, 可见DNA纤丝; 质体形态多样, 片层明显, 内含淀粉粒。受精前, 其内的胞质最多, 细胞代谢最强; 至合子的合点端建成蜂窝状细胞壁且游离核已形成时, 宿存助细胞开始退化; 至合子后期退化完全。另外, 2个助细胞的丝状器一直存在于胚囊的珠孔端, 直至两细胞均退化, 而且在其附近一直有退化的珠心细胞出现。推测宿存助细胞做为传递细胞, 为胚囊的发育吸收并转输营养。
关于助细胞退化的机制, 尚没有明确答案。助细胞在花粉管进入后退化的情况, 认为是花粉管的进入物理性破坏助细胞, 直接导致其退化[ 25]; 助细胞在授粉后且花粉管进入之前退化的情况, 认为助细胞退化依赖花粉管诱导, 如Jensen等[ 14]通过离体实验推测花粉管在花柱中生长时产生的信号使赤霉素增加, 并扩散到胚囊, 诱导助细胞退化; 烟草中研究发现助细胞退化大都发生在授粉后42~48 h, 即花粉管进入子房而尚未到达胚囊之前, 如果不授粉或授粉后36 h以前切断花柱阻止花粉管进入子房, 助细胞不退化, 表明助细胞退化由花粉管诱导, 并且受花粉管进入子房后的近距离调控[ 15]。然而在小麦和珍珠谷的研究中, 传粉前助细胞即开始退化, 说明助细胞的退化与传粉和花粉管无关[ 12, 13]。而且在珍珠谷中发现早期钙主要存在于小液泡中, 随退化进行, 液泡中的钙进入细胞质, 超常的高钙含量导致细胞器退化, 进而整个细胞退化, 表明助细胞退化受钙调节。Wu和Cheung[ 26]用细胞程序死亡的观点探讨助细胞退化问题, 亦认为钙介导细胞凋亡。本研究中栽培甜菜在花蕾时期(授粉前)助细胞即开始退化, 待花蕾刚开放(授粉前)助细胞即完全退化, 可见其助细胞退化与授粉和花粉管生长无关, 推测可能与细胞程序性死亡有关, 而引发凋亡的信号尚有待进一步研究。
在栽培甜菜助细胞的退化进程中, 一个助细胞的退化起始于花蕾时期, 待花蕾刚开放(授粉前)时完全退化, 仅余退化残迹。可见助细胞退化与授粉和花粉管生长无关。宿存助细胞存在时间较长, 在合子建成蜂窝状细胞壁且胚乳游离核已形成时开始退化, 至合子分裂前完全退化。一个助细胞退化后, 另一个助细胞(宿存助细胞)内的胞质逐渐增多, 细胞器功能趋于活跃, 呈现活跃的细胞代谢活性, 至卵细胞受精前达到顶峰。丝状器一直存在至两助细胞完全退化, 丝状器旁有退化的珠心细胞出现。表明宿存助细胞做为传递细胞, 为胚囊的发育吸收并转输营养。
Explanation of plate:
The micropyle is in upward position in each panel. CC: central cell; CW: cell wall; DSy: degenerated synergid; EC: egg cell; FA: filiform apparatus; G: golgi body; L: lipid body; M: mitochondrium; N: nucleus; P: plastid; PSy: persistant synergid; RER: rough endoplasmic reticulum; S: starch grain; Sy: synergid; V: vacuole; Ve: vesicle; Z: zygote.
图版说明:
所有图片均是珠孔端朝上。CC: 中央细胞; CW: 细胞壁; DSy: 退化助细胞; EC: 卵细胞; FA: 丝状器; G: 高尔基体; L: 脂体; M: 线粒体; N: 核; P: 质体; PSy: 宿存助细胞; RER: 粗面内质网; S: 淀粉粒; Sy: 助细胞; V: 液泡; Ve: 小泡; Z: 合子。
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|



