* 通讯作者(Corresponding author): 申家恒, E-mail:hsdshenjiaheng@163.com
第一作者联系方式: E-mail:hsdliwei@163.com
应用透射电镜技术比较栽培甜菜(
The ultrastructural changes of central cell before and after fertilization were observed with TEM to provide more information for reproductive biology of sugar beet and relative research. The results indicated that the changes of central cell before and after fertilization mainly occurred in nucleus and cytoplasm around. Fusion of polar nuclei took place early at bud stage. The nucleolus of secondary nucleus was bipolar with notable fibrous and particle core. Sometimes, there was extra small nucleolus and nucleolus vacuole. Cytoplasm surrounding secondary nucleus was rich in organelles, including mitochondria, plastids with or without starch grain, Golgi body, ribosomes and rough endoplasmic reticulum (rER). After fertilization, a striking feature of cytoplasm surrounding nucleus was the well developed amoeboid plastids, which were numerous and various, filling with starch gains. Soon after that, primary endosperm nucleus divided into two free nuclei. During division, nucleolus disappeared and nuclear membrane disintegrated into small vesicles. Also, there was an obvious decrease in rER and an increase in smooth endoplasmic reticulum (sER). Overall, central cell was active before and after fertilization.
中央细胞是被子植物胚囊中体积最大的细胞, 含有2个极核或一个次生核, 受精后形成初生胚乳核, 进而发育为胚乳。对中央细胞受精前后超微结构的研究, 将为进一步开展生殖生物学和分子细胞生物学研究发挥重要作用。迄今, 已在油菜[ 1]、向日葵[ 2, 3]、玉米[ 4]、水稻[ 5]等一些植物中报道了中央细胞受精前后的超微结构特点[ 6, 7, 8, 9]。然而, 对同一种植物中央细胞受精前后超微结构的对比研究较少, 仅在打碗花[ 10]、荠菜[ 11]和向日葵[ 12]中描述过。栽培甜菜为重要的糖料作物, 目前对其生殖生物学的亚显微水平研究包括大孢子发生[ 13]、雌配子体发育[ 14]、花粉[ 15, 16]、成熟胚囊[ 17]、卵细胞、合子及二细胞原胚[ 18]; 显微水平的研究有大、小孢子发生和雌、雄配子体发育[ 19]及受精作用[ 20]。本研究在前人工作基础上详细描述了栽培甜菜中央细胞受精前后的超微结构变化, 包括次生核、初生胚乳核及其分裂、胚乳游离核时期的超微结构特点, 以期完善甜菜生殖生物学研究, 并为相关研究提供基础资料。
将栽培甜菜双丰1号( Beta vulgaris Shuangfeng 1)种植于黑龙江大学植物园。2004—2006年, 每年6月末至7月中旬, 取直径为1.5~3.0 mm的花蕾及刚开放的小花, 剥离胚珠并迅速投入3%戊二醛(pH 7.2二甲胂酸钠缓冲液配制)固定; 每天清晨8:00对刚开放的花朵人工异花授粉, 授粉后13~27 h内, 每2 h取雌蕊50个, 剥离胚珠并用3%戊二醛固定。以缓冲液冲洗4次, 再用1%锇酸室温下固定2~4 h, 缓冲液冲洗后, 系列乙醇脱水, 经环氧丙烷置换, Spurr树脂渗透包埋。使用Leica Ultracut R型超薄切片机切片(厚度70 nm), 经醋酸双氧铀-柠檬酸铅双重染色后, 在JEOL-1230型透射电镜下观察并拍照。
栽培甜菜雌配子体发育类型为蓼型。成熟的中央细胞高度液泡化, 通过液泡的细胞质索使核周区细胞质与周缘细胞质连接。中央细胞在受精前后分别经历2个极核、次生核、初生胚乳核和胚乳游离核阶段, 其超微结构发生如下变化。
胚囊细胞化时期, 中央细胞内存在上、下2个极核(图版I-A)。此时期, 胚囊珠孔端细胞呈楔形, 尚未分化, 无法区分卵细胞和助细胞(图版I-E); 合点端存在3个反足细胞(图版I-I); 中间为中央细胞, 其上极核紧邻珠孔端细胞(图版I-B, E), 下极核靠近反足细胞(图版I-H, I)。各细胞之间均以完整的细胞壁相隔, 壁上存在胞间连丝(图版I-F, G, I)。胞质中粗面内质网的潴泡呈索状延伸于细胞壁或细胞核旁(图版I-C, D, G)。
中央细胞的2个极核融合较早, 在花蕾时期——胚囊刚成熟时即以次生核形式存在(图版II-A)。次生核的电子密度高, 被胞质索包围, 悬在细胞中央。核呈球形, 核仁双相, 内部由一些无定形物质及纤维状物质交织在一起, 而外部是类似核糖体大小的致密颗粒(图版II-B)。核周围的胞质浓厚, 细胞器丰富。线粒体数目众多, 且内嵴明显, 偶见DNA纤丝; 质体小, 不易分辨; 粗面内质网的潴泡相互联结, 大量存在于胞质中; 核糖体浓度大(图版II-C, D)。
花蕾刚开放时期, 胚囊纵向延长, 形成盲囊(图版II-E)。次生核呈橄榄形, 贴在中央细胞的一侧(图版II-F, G)。核大且双相核仁异常显著, 外部的致密颗粒大量存在(图版II-G, H), 少量颗粒物质正在向核外输出(图版II-J)。核周围的胞质变稀薄, 高尔基体活跃地分泌小泡(图版II-I)。
开花后的胚囊中(图版III-A), 次生核内出现分散的染色质和小的核仁液泡(图版III-B)。核周围的细胞质明显增多, 细胞器也随之增多且功能活跃, 包括线粒体、质体、粗面内质网和高尔基体(图版III-C~F)。
授粉后13 h (受精前)的胚囊中(图版III-G, H), 次生核呈不规则形状(图版III-I), 连续切片显示次生核内除一个大核仁外还有2个额外小核仁, 大、小核仁内均存在核仁液泡(图版III-J)。质体中出现淀粉粒(图版III-K, M); 线粒体内可见DNA纤丝(图版III-L)。整个次生核阶段, 中央细胞呈现代谢活跃状态。
授粉后约17 h, 中央细胞受精形成初生胚乳核(图版IV-A)。初生胚乳核的体积较次生核明显增大, 为浓厚的胞质所包围(图版IV-B)。初生胚乳核的核仁显著, 核膜不规则, 呈裂瓣状, 大大地增加了核膜的表面积; 不规则的核膜包围部分胞质, 其中线粒体多且内嵴明显(图版IV-C, D)。核周围的胞质中富含细胞器, 包括线粒体、质体、粗面内质网、高尔基体, 且功能活跃(图版IV-C)。质体较受精前变化大, 形成众多变形质体, 形态多样, 呈哑铃型、弓形、杯状等, 电子染色深, 有的含淀粉核(图版IV-E), 有的杯状质体包围线粒体。从超微结构上看, 初生胚乳核阶段细胞的代谢相当活跃。
大多数被子植物在胚囊成熟过程中, 中央细胞的2个极核相互靠近, 并最终在受精前融合成为一个二倍体的次生核[ 21], 如向日葵[ 3]、荠菜[ 11]等。有些植物的极核在受精前已经开始局部融合, 直到受精时才完成融合, 如打碗花[ 10]、棉花[ 22]、小麦[ 23]、大豆[ 24]等。本研究中, 中央细胞的2个极核融合较早, 在花蕾时期(胚囊刚形成时)的胚囊中即以次生核状态存在。这与申家恒等[ 19]在甜菜光镜水平研究中“开花前, 两极核已融合为次生核”的描述相同, 也与Bruun[ 17]在甜菜成熟超微结构研究中所认为的“两极核的融合似乎发生于胚囊成熟过程的早期”相吻合。
中央细胞受精前后的变化主要体现在核及核周围胞质上。中央细胞的次生核具明显的双相核仁, 内部由无定形物质及纤维状物质交织在一起形成大而浓密的纤维区, 外部由类似核糖体大小的致密颗粒组成明显的颗粒状区, 推测有大量的前核糖体分子积累。这与油菜[ 1]和打碗花[ 10]中报道的极核的核仁相似。有的次生核具额外的小核仁, 且有明显的核仁液泡。而次生核周围的胞质, 正如其他植物中报道的一样[ 2, 3, 4, 5, 22, 25, 26], 其内细胞器丰富且功能活跃, 含大量的线粒体、质体(含或不含淀粉粒)、高尔基体、粗面内质网和核糖体。由超微结构特点来看, 栽培甜菜的中央细胞是代谢非常活跃的细胞, 这无疑与其受精前的营养功能和为受精后胚乳的发育作准备有关[ 2]。
次生核与精核融合后形成体积较大的初生胚乳核, 核膜不规则, 呈裂瓣状, 包围部分胞质, 此胞质中含线粒体多且内嵴明显, 而杯状变形质体也将线粒体包含其内, 其功能和意义尚不清楚。初生胚乳核经核分裂形成2个较小的胚乳游离核。我们仅捕捉到初生胚乳核分裂的中期、后期相, 发现核仁消失, 核膜崩解为许多囊泡状结构, 粗面内质网减少, 滑面内质网增加。这与矮生菜豆胚乳细胞形成时的描述相似[ 9]。囊泡状结构的成分及是否参与核膜的重建尚待进一步研究。
中央细胞受精后, 初生胚乳核及胚乳游离核周围胞质中, 变化最大的是质体, 受精后形成众多变形质体, 形态多样, 呈哑铃型、弓形、杯状等, 电子染色深, 有的含淀粉核; 而其他细胞器未见明显变化。这与荠菜[ 11]相似, 但与玉米[ 6]和矮牵牛[ 7]中描述的受精后内质网、核糖体、高尔基体大量增加不同, 推测与栽培甜菜中央细胞次生核阶段本身代谢已经相当活跃有关。
所有图片均为珠孔端朝上。A: 反足细胞; CC: 中央细胞; Chr: 染色体; CW: 细胞壁; E: 卵细胞; End: 胚乳; G: 高尔基体; Gv: 高尔基体的小泡; M: 线粒体; N: 核; Nu: 核仁; Nv: 核仁液泡; P: 质体; PN: 极核; RER: 粗面内质网; S: 淀粉粒; SER: 滑面内质网; SNu: 次生核; Sy: 助细胞; V: 液泡; Ve: 小泡; Zy: 合子。
Explaination of plate
The micropyle is upward position in each panel. A: antipodal cell; CC: central cell; Chr: chromosome; CW: cell wall; E: egg; End: endosperm; G: golgi body; Gv: golgi vesicle; M: mitochondrium; N: nucleus; Nu: nucleolus; Nv: nucleolus vacuole; P: plastid; PN: polar nucleus; RER: rough endoplasmic reticulum; S: starch grain; SER: smooth endoplasmic reticulum; SNu: secondary nucleus; Sy: synergid; V: vacuole; Ve: vesicle; Zy:zygote.
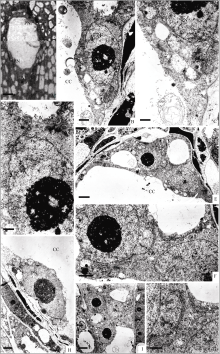
 | 图版II 花蕾时期次生核阶段中央细胞的结构A: 花蕾时期胚囊的半薄切片, 示中央细胞的次生核(Bar = 5 μm); B~D: 花蕾时期中央细胞的超微结构, 图B示次生核(Bar = 2 μm); 图C、D示次生核周围胞质中的线粒体、内质网和高尔基体(Bar = 500 nm); E~F: 花蕾刚开放时胚囊的相邻半薄切片, 图E示反足细胞, 图F示中央细胞的次生核(Bar = 10 μm); G~J: 花蕾刚开放时中央细胞的超微结构, 图G示次生核(Bar = 2 μm); 图H示双相核仁(Bar = 1 μm); 图I示次生核周围胞质中的线粒体、内质网和高尔基体(Bar = 500 nm); 图J箭头示颗粒状物质正向核外输出(Bar = 200 nm)。Plate II Structure of central cell at secondary nucleus stage during bud periodA: semi-thin section of embryo sac during bud period, showing secondary nucleus (Bar = 5 μm); B–D: ultrastructure of central cell during bud period. B: secondary nucleus (Bar = 2 μm); C–D: mitochondria, endoplasmic reticulum and the Golgi around secondary nuclear (Bar = 500 nm); E–F: semi-thin sections of embryo sac at the beginning of flowering; E: antipodal cell; F: secondary nucleus (Bar = 10 μm); G–J: ultrastructure of central cell at the beginning of flowering. G: secondary nucleus (Bar = 2 μm); H: bipolar nucleolus (Bar = 1 μm); I: mito-chondria, endoplasmic reticulum and the Golgi around secondary nuclear (Bar = 500 nm); J: particle material is being output from nuclear(Bar = 200 nm). |
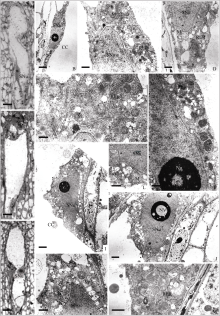 | 图版III 开花后次生核阶段中央细胞的结构A: 开花后胚囊的半薄切片, 示中央细胞的次生核(Bar = 10 μm); B~F: 开花后中央细胞的超微结构, 图B示次生核(Bar = 5 μm); 图C~F示核周围胞质中的线粒体、内质网、高尔基体和质体(图C、D、F, Bar = 1 μm; 图E, Bar = 500 nm); G~H: 授粉后约13 h 胚囊的相邻半薄切片, 示中央细胞的次生核(Bar = 10 μm); I~M: 授粉后约13 h中央细胞的超微结构, 图I、J为相邻切片, 示次生核及核仁液泡(Bar = 2 μm); K~M: 核周围胞质中的内质网、线粒体和质体(Bar = 1 μm)。Plate III Structure of central cell at secondary nucleus stage after floweringA: semi-thin section of embryo sac after flowering, showing secondary nucleus (Bar = 10 μm); B–F: ultrastructure of central cell after flowering; B: secondary nucleus (Bar = 5 μm); C–F: mitochondria, endoplasmic reticulum, the Golgi and plastids around secondary nuclear (C, D, F, Bar = 1 μm; E, Bar = 500 nm); G–H: semi-thin sections of embryo sac approximately 13 hours after pollination, showing secondary nucleus (Bar = 10 μm); I–M: ultrastructure of central cell approximately 13 hours after pollination; I–J: secondary nucleus and vacuole of nucleolus(adjacent sections, Bar = 2 μm); K–M: endoplasmic reticulum, mitochondria and plastids around secondary nuclear (Bar = 1 μm). |
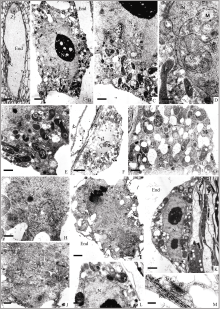 | 图版IV 初生胚乳核及胚乳游离核阶段中央细胞的结构A: 授粉后约17 h胚囊的半薄切片, 示初生胚乳核(Bar = 10 μm); B~E: 授粉后约17 h中央细胞的超微结构, 图B示初生胚乳核(Bar = 2 μm); C~D: 核膜包围部分细胞质(图C, Bar = 1 μm; 图D, Bar = 500 nm); E: 核周围胞质中的质体(Bar = 1 μm)。F~H: 初生胚乳核分裂中期的超微结构, 图F示初生胚乳核分裂中期(Bar = 5 μm); 图G示周围胞质中的质体、线粒体和高尔基体(Bar = 1 μm); 图H示核膜崩解为许多囊泡状结构(Bar = 500 nm); I~J: 初生胚乳核分裂后期的超微结构, 图I示初生胚乳核分裂后期(Bar = 2 μm); 图J示周围胞质中滑面内质网增多(Bar = 500 nm); K~M: 胚乳游离核阶段的超微结构, 图K示胚乳游离核(Bar = 2 μm); 图L示核周围胞质, 质体含淀粉粒(Bar = 1 μm); 图M示核周围胞质中的内质网, 高尔基体分泌小泡(Bar = 200 nm)。Plate IV Structure of central cell at the stage of primary endosperm nucleus and free nucleiA: semi-thin section of embryo sac approximately 17 hours after pollination, showing primary endosperm nucleus (Bar = 10 μm). B–E: Ultrastructure of central cell approximately 17 hours after pollination; B: primary endosperm nucleus (Bar = 2 μm); C–D: cytoplasm surrounded by nuclear membrane (C, Bar = 1 μm; D, Bar = 500 nm); E: plastid around primary endosperm nuclear (Bar=1 μm); F–H: ultrastructure of primary endosperm nucleus during metaphase division; F: metaphase division (Bar = 5 μm); G: plastids, mitochondria and Golgi in the surrounding cytoplasm (Bar = 1 μm); H: nuclear membrane disintegrated into small vesicles (Bar = 500 nm); I–J: ultrastructure of primary endosperm nucleus during anaphase; I: anaphase division (Bar = 2 μm); J: smooth endoplasmic reticulum increase (Bar = 500 nm); K–M: ultrastructure of free nuclei stage; K: free nuclei (Bar = 2 μm); L: cytoplasm surrounding nucleus, plastids containing starch grain (Bar =1 μm); M: endoplasmic reticulum and Golgi secretory vesicles (Bar = 200 nm). |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|