第一作者联系方式: 杨宗举, E-mail: zongjuyang@163.com; 闫蕾, E-mail: yanlei2723@126.com, Tel: 010-82105851
光敏色素是一类红光/远红光受体, 它们在植物体内有非活性形式的红光吸收型(Pr)和活性形式远红光吸收型(Pfr) 2种状态, 通常其活性形式负责调控植物的种子萌发、株高、开花时间和避荫性等生长发育过程。在禾本科中, 光敏色素只有 PHYA、 PHYB和 PHYC三个基因亚家族, 古四倍体化造成的玉米光敏色素基因有6个成员, 即 PHYA1、 PHYA2、 PHYB1、 PHYB2、 PHYC1和 PHYC2。光敏色素A参与抑制下胚轴的伸长、促进张开子叶和花青素的积累、阻断持续远红光条件下的变绿。为了评价 ZmPHYA1和 ZmPHYA2对光的响应能力及其功能差异, 本研究采用实时定量PCR技术分析玉米自交系B73和Mo17中 ZmPHYA1和 ZmPHYA2对不同光照处理响应的表达模式。结果表明玉米光敏色素A主要在叶片和花丝中表达, 并且 ZmPHYA1转录丰度是 ZmPHYA2的2~8倍; 玉米自交系B73和Mo17中胚轴在黑暗、远红光和蓝光条件下较红光和白光下更长。 ZmPHYA1和 ZmPHYA2的转录水平在持续远红光和蓝光条件下均较高; 并且均较迅速响应黑暗到远红光和蓝光光质转换, 但是前者的丰度显著高于后者, ZmPHYA1在远红光下更重要, 而 ZmPHYA2在蓝光下更重要。 ZmPHYA1和 ZmPHYA2同样响应于黑暗到红光和白光的转换, 并且 ZmPHYA1和 ZmPHYA2表达模式基本一致。 ZmPHYA1和 ZmPHYA2的表达均能响应长日照和短日照处理, 但是 ZmPHYA1转录丰度高于 ZmPHYA2的2~5倍。以上结果表明, ZmPHYA1和 ZmPHYA2的转录能有效地响应各种光处理, 可能 ZmPHYA1在作物改良上比 ZmPHYA2更有效。本研究为进一步了解 ZmPHYA1和 ZmPHYA2基因功能以及评价二者的光反应能力提供了理论基础。
Plant phytochromes are a family of red/far-red light photoreceptors, which have two forms in plant: inactive red light absorbing form (Pr) and active far-red light absorbing form (Pfr). During plant growth and developmental processes, phytochromes play pivotal roles in regulations of seed germination, plant height, flowering time, and shade-avoidance. In the grasses, three subfamilies are present: PHYA, PHYB and PHYC. In maize, an ancient genome duplication has increased the family member to six: PHYA1, PHYA2, PHYB1, PHYB2, PHYC1, and PHYC2. Phytochrome A facilitates the inhibition of hypocotyl elongation, opening of the apical hook, expansion of cotyledons, accumulation of anthocyanin and blocking of greening by continuous FR (FRc) light. In order to evaluate the light response capability and difference of transcription abundance between ZmPHYA1 and ZmPHYA2, we employed quantitative real-time PCR (qRT-PCR) assay to investigate the expression patterns of ZmPHYA1 and ZmPHYA2 in the inbred line B73 and Mo17 with different light treatments. The results indicated that both ZmPHYA1 and ZmPHYA2 had a high expression level in leaf and silk, and the transcription abundance of ZmPHYA1 was 2-8 times higher than that of ZmPHYA2. Inbred lines of both B73 and Mo17 possessed longer mesocotyls in dark, far-red and blue light conditions than in red or white light conditions. Both ZmPHYA1 and ZmPHYA2 had a high expression level in far-red and blue lights and rapidly responded to dark-to-far-red and dark-to-blue transitions. ZmPHYA1 was more important under far-red light, so was ZmPHYA2 in blue light. Both of the genes could rapidly respond to transitions from dark to red or white light with similar expression pattern. The both genes also respond to long-day or short-day treatments, however the transcription abundance of ZmPHYA1was 2-5 times higher than that of ZmPHYA2during the treatments. All the results suggested that the transcription of both ZmPHYA1 and ZmPHYA2 could rapidly responded to different light treatments; ZmPHYA1might be more effective than ZmPHYA2 in crop improvement. Our results provide a theoretical basis for the function study and evaluation of light response ability for both ZmPHYA1 and ZmPHYA2.
植物生长发育过程会受到来自环境的生物及非生物因素影响[1], 其中光作为重要的非生物环境因子, 对植物整个生命过程起至关重要的作用[2]。光不仅是植物光合作用的能量来源, 而且作为环境信号参与调控种子萌发、幼苗去黄化、叶片展开、下胚轴伸长、向光性、气孔开关、叶绿体移动、避荫性、节律、开花等生长和发育过程[2, 3, 4, 5, 6]。植物的光受体系统负责感知光的强度、波长、节律和方向[6, 7, 8, 9, 10, 11], 高等植物至少存在3类光受体系统, 以满足它们对不同波长信号(红光/远红光、蓝光/紫外光A, 以及紫外光B)的吸收[12], 其中光敏色素是红光/远红光(600~750 mm)的受体[13]。模式植物拟南芥具有5个光敏色素基因(PHYA-PHYE)[14], 它们在植物生长和发育过程中明确分工, 又功能冗余[2]。光敏色素A (phyA)和光敏色素B (phyB)均参与萌发的调节, 但前者主要是负责极低辐照反应(very-low-fluence response, VLFR, 0.1~1.0 µ mol m-2 s-1), 而后者主要负责低辐照反应(low-fluence response, LFR, 1~1000 µ mol m-2 s-1)[15, 16, 17]。光敏色素A属于光不稳定类型[18], 在黑暗和远红光条件下转录和蛋白丰度较高, 而在红光和白光下蛋白质稳定性极差, 转录水平也很低[19]。拟南芥的光敏色素A不但参与远红光信号途径, 而且介导红光和蓝光信号转导[20]。研究表明, phyA在植物整个生命过程中起重要作用[21]。黑暗条件下丰度最高的光受体phyA对萌发种子光形态建成的开启起主要作用; 露出土壤后的幼苗通过phyA感知周围因遮盖导致的弱辐照, 启动极低辐照反应来完成最初的去黄化反应[22]。phyA在黑暗条件下是稳定的红光吸收型(Pr), 受光激活后变为极易被降解的远红光
吸收型(Pfr)[23, 24, 25]。phyA突变体在短日照处理条件下开花延迟、叶片增多, 表明phyA参与感受光周期的变化[26]。在长日照条件下, phyA通过增加CONSTANS (CO)蛋白的稳定性来提高FLOWERING LOCUS T (FT)的表达, 进而加速开花[27]。phyA不仅在促进种子萌发和幼苗去黄化过程发挥重要作用[28], 而且通过增强phyB的作用来抑制植物的避荫性反应[29]。马铃薯的phyA通过促进体内花青素合成和蔗糖磷酸合成酶活性, 来增强幼苗早期对周围环境感知的能力[30]。水稻的phyA在长日照条件下延迟开花, 却在短日照条件下促进开花[31]。
通过修饰作物的光敏色素途径可以改良株高、增强光反应能力和提高产量。在水稻中过量表达拟南芥PHYA基因, 尽管没有导致转基因植株节数的变化, 但是节间却明显缩短, 导致成株高度显著降低[32]。在马铃薯中转入拟南芥PHYB基因, 转基因植株在高密度栽培条件(20株 m-2)下, 节间和叶柄缩短而株高明显降低, 叶绿素含量高而光合作用增强, 单株块茎产量得到显著提高[33, 34, 35]。在烟草中过量表达燕麦PHYA基因可以增加叶面积指数[36]。最新的研究表明, 在长日照条件下小麦PHYC的缺失使生物钟及光周期基因表达发生改变, 因而开花延迟[37]。
迄今, 人们对于双子叶植物模式植物拟南芥的光敏色素研究较为深入, 但对于单子叶植物光敏色素的研究还鲜有报道。在禾本科植物中, 光敏色素基因只存在3个亚家族: PHYA、PHYB和PHYC[40, 41]。玉米的古四倍体化过程导致其产生光敏色素A有2个拷贝, ZmPHYA1和ZmPHYA2, 它们之间是否存在功能分化, 以及二者之间的相互关系, 一直引起我们的关注。本研究以玉米自交系B73和Mo17为材料, 测量不同光质条件下玉米中胚轴长度; 通过实时定量PCR技术研究不同光照处理下ZmPHYA1和ZmPHYA2的表达差异, 了解它们的表达模式, 为评价二者的光反应能力及在玉米改良中的价值提供科学依据。
1.1.1 试验材料 选用玉米自交系B73和Mo17 (本实验室保存)。
1.1.2 器官特异性表达样品的准备 将自交系B73的种子在北京地区4月下旬播种于花盆中自然条件下生长60 d, 分别取成株的根、茎、叶、叶枕、叶鞘、花丝、花柄、雄花、苞叶和幼穗。
1.1.3 各种持续光质处理 将玉米种子消毒杀菌后, 平放在湿润滤纸的培养皿中28℃催芽3 d, 挑选萌动一致的种子播于装有培养土塑料小盆(长宽高均为8.5 cm)中, 每小盆播9粒, 所有玉米幼苗均生长在22℃。分别放黑暗(Dk)、远红光(FR, 0.5 µ mol m-2 s-1)、红光(R, 22.3 µ mol m-2 s-1)、蓝光(B, 13 µ mol m-2 s-1)和白光(WL, 17 µ mol m-2 s-1)培养箱中生长13 d。
1.1.4 黑暗转换各种光质 将幼苗自黑暗中生长13 d, 分别转入以上各种光质条件下, 0.25、0.5、1、2、4、8、12和24 h后, 分别取幼苗地上部为试材。
1.1.5 长日照和短日照处理 玉米幼苗在22℃长日(LD, 16 h光照/8 h黑暗)或短日(SD, 8 h光照/16 h黑暗)条件下中生长13 d, 每隔2 h取一次样。
用TRIzol (Invitrogen, USA)法提取各种处理的玉米幼苗总RNA, 经DNase I (RNase-free, TaKaRa大连公司)处理后作为模板, Oligo-dT18为引物, 利用RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific公司)反转录成cDNA备用。
以不同处理的B73玉米的cDNA第1链为模板, 玉米Tubulin基因为内参, 测定ZmPHYA1和ZmPHYA2的相对表达量。ZmPHYA1和ZmPHYA2与Tubulin的序列分别来源于PLAZA网站和NCBI网站, 利用Primer Premier 5.0软件设计荧光定量PCR引物(表1)。荧光定量PCR仪为Roche 480 (Roche, 瑞士), PCR程序为95℃预变性30 s, 再进行以下循环: 95℃变性5 s, 60℃退火20 s, 72℃延伸10 s, 共进行50个循环; 然后60~95℃绘制溶解曲线。定量PCR试剂为SYBR Premix Ex Taq II (TaKaRa大连公司), 按照商家使用说明操作。采用2-Δ Δ CT的方法计算结果[42]。经3次独立的生物学重复, 并依此计算标准差。采用SAS9.2软件对数据进行方差及相关分析, 用Duncan’ s新复极差法进行多重比较, 并采用Microsoft Excel 2010软件绘图。
| 表1 qRT-PCR所用引物 Table 1 Primers for qRT-PCR |
玉米光敏色素A基因在根、茎、叶、叶枕、叶鞘、花丝、花柄、雄花、苞叶和幼穗中均有表达, 尤其在叶片和花丝中的表达量较高, 其中ZmPHYA1在叶片和花丝中分别为在根中的6.5倍和10.9倍, 推测叶片和花丝是玉米光敏色素A主要起作用的部位。从图1可知, 根、茎、叶、叶枕、叶鞘、花丝、花柄和苞叶中ZmPHYA1的转录丰度约为ZmPHYA2的4~10倍, 雄花和幼穗中ZmPHYA1的转录丰度约为ZmPHYA2的1.5倍和3.0倍。ZmPHYA1在各器官中的表达丰度均明显高于ZmPHYA2, 暗示ZmPHYA1可能比ZmPHYA2有更重要的作用。
采用玉米中胚轴作为衡量其光形态建成程度的指标, 为了解它的伸长与光质的关系, 测量了在不同光质持续照射条件下玉米自交系B73和Mo17的中胚轴长度。在黑暗条件下因黄化反应, 中胚轴明显长于其他光质处理(图2)。远红光、红光、蓝光和白光均可以抑制玉米中胚轴伸长, 并且红光和白光条件下对中胚轴的抑制作用较远红光和蓝光更为明显。可见, 玉米中胚轴伸长是响应各种光质处理的。
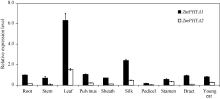 | 图1 ZmPHYA1和ZmPHYA2器官特异性表达的定量RT-PCR分析 |
分别取玉米自交系B73(60 d)的不同器官(包括根、茎、叶、叶枕、叶鞘、花丝、花柄、雄花、苞叶和幼穗)用于qRT-PCR分析。把ZmPHYA1在根中的表达丰度设为对照, 并且将该ZmPHYA1/Tubulin设为1。柱状图为3次独立的生物学重复ZmPHYA/Tubulin比值的平均值, 误差线代表标准差。
Different organs of 60-day-old maize inbred line B73, including root, stem, leaf, pulvinus, sheath, silk, pedicel, stamen, bract and young ear were used in qRT-PCR assay. The transcription abundance of ZmPHYA1 in root was set as the control, and the ratio of ZmPHYA1/Tubulin in root was set as 1. Each column shows the mean relative expression of ZmPHYA/Tubulin from three biological repeats. Error bars indicate the standard deviation.
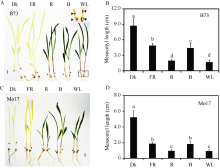 | 图2 各种持续光质条件下B73和Mo17中胚轴长度Fig. 2 Mesocotyl lengths of B73 and Mo17 under different continuous light conditions |
玉米自交系B73和Mo17的幼苗在黑暗(Dk)、持续远红光(FR, 1.9 µ mol m-2 s-1)、持续红光(R, 22.3 µ mol m-2 s-1)、持续蓝光(B, 13.0 µ mol m-2 s-1)和持续白光(WL, 17.0 µ mol m-2 s-1)下生长13 d。以软件Image J测量中胚轴长度。A:玉米自交系B73表型图, 小图为黑框的放大图。B:玉米自交系B73中胚轴长度。C:玉米自交系Mo17表型图。D:玉米自交系Mo17中胚轴长度。图中标尺为10 mm, 箭头表示中胚轴位置。柱值为中胚轴长度的平均值, 误差线代表标准差。柱上不同或相同的小写字母表示不同光照条件下幼苗的中胚轴长度经F测验(ANOVA软件)达到或未达到5%差异水平(P < 0.05)。
The seedlings of maize inbred line B73 and Mo17 were grown in the dark (Dk), continuous far-red light (FR, 1.9 µ mol m-2 s-1), red light (R, 22.3 µ mol m-2 s-1), blue light (B, 13.0 µ mol m-2 s-1) and white light (WL, 17.0 µ mol m-2 s-1) for 13 d, respectively. Measurement of mesocotyl length by Image J. A: phenotype of the inbred line B73, the small figure is the magnification of the black box. B: mesocotyl length of the inbred line B73. C: phenotype of the inbred line Mo17. D: mesocotyl length of the inbred line Mo17. Bars = 10 mm. The arrows represent mesocotyl position. Each column shows the mean mesocotyl length of three biological repeats. Error bars indicate the standard deviation. Bars represented by the same lower case letter are not significant different (P < 0.05) among seedlings grown in different light conditions according to F-test in an analysis of variance (ANOVA), while those by different lower case letters are significant different.
图3-A显示ZmPHYA1在红光和白光下的转录丰度很低, 仅为自身黑暗中的30%和40%; 远红光条件下, ZmPHYA1的转录丰度最高, 约为自身黑暗中的1.4倍; 而蓝光下的表达丰度与黑暗条件下相似。ZmPHYA2在红光和白光中表达丰度也非常低(图3-B), 在蓝光下的转录丰度约为自身黑暗中的1.3倍; 而远红光下ZmPHYA2的转录丰度仅为其在黑暗中的80%。持续光条件下的转录表达分析结果显示, ZmPHYA1和ZmPHYA2均受到红光和白光的强烈抑制, 这与拟南芥的PHYA基因结果类似, 可能与它们编码的蛋白质在光下不稳定有关。另外从二者在远红光和蓝光下的转录丰度推测, 2个基因在远红光和蓝光中均具有作用; ZmPHYA1在远红光下更重要, 而ZmPHYA2在蓝光下更重要。
 | 图3 各种持续光质条件下ZmPHYA1和ZmPHYA2转录表达的定量RT-PCR分析Fig. 3 qRT-PCR assay of ZmPHYA1 and ZmPHYA2 expression under different continuous light conditions |
玉米自交系B73的幼苗在黑暗(Dk)、持续远红光(FR, 1.9 µ mol m-2 s-1)、持续红光(R, 22.3 µ mol m-2 s-1)、持续蓝光(B, 13.0 µ mol m-2 s-1)和持续白光(WL, 17.0 µ mol m-2 s-1)下生长13 d。把ZmPHYA1在黑暗条件下的表达丰度设为对照, 并且将该ZmPHYA1/Tubulin设为1。柱值代表3次独立的生物学重复ZmPHYA/Tubulin表达比值的平均值, 误差线代表标准差。
The seedlings of maize inbred line B73 were grown in dark (Dk), continuous far-red light (FR, 1.9 µ mol m-2 s-1), continuous red light (R, 22.3 µ mol m-2 s-1), continuous blue light (B, 13.0 µ mol m-2 s-1) and continuous white light (WL, 17.0 µ mol m-2 s-1) for 13 d. The transcription abundance ofZmPHYA1in the dark was set as the control, and the ratio of ZmPHYA1/Tubulin in the dark was set as 1. Each column shows the mean relative expression of ZmPHYA/Tubulin from three biological repeats. Error bars indicate the standard deviation.
我们将黑暗中生长13 d的B73幼苗分别转入到远红光、蓝光、红光和白光下0、0.25、0.5、1、2、4、8、12和24 h, 来进一步探究ZmPHYA1和ZmPHYA2转录丰度对不同光质转换的响应。ZmPHYA1的转录能迅速响应黑暗到远红光的转换, 0.25 h内其转录水平上升到自身黑暗中的1.9倍, 0.5 h内继续上升到自身黑暗中的4.5倍, 之后迅速上升并在1 h达到峰值(黑暗中的18倍); 但在2 h、4 h和8 h分别下降到峰值的70%、60%和6%, 其较低的转录水平一直维持到12 h; 在远红光处理24 h后, 其转录水平又回升到自身黑暗中的3.6倍(图4-A)。ZmPHYA2在黑暗到远红光的转换最初0.5 h之内与ZmPHYA1的转录水平相当, 属于较平缓的上升, 但在1 h上升到仅为黑暗中ZmPHYA1的3.4倍的峰值; 随后缓慢下降, 8 h时达到超低值(黑暗中ZmPHYA1的2%), 并且其转录在24 h内均维持较低水平(黑暗中ZmPHYA1的2%~10%)。由此可见, ZmPHYA1和ZmPHYA2均能迅速响应远红光; ZmPHYA2的转录在24 h内只有一个峰值, 上升和下降均较缓慢; 而ZmPHYA1的转录不但迅速响应黑暗到远红光的转换, 并且峰值达到了自身黑暗中的18倍。这些结果暗示ZmPHYA2在持续远红光中起重要作用, 而ZmPHYA1在对远红光光质转换的响应中更重要。
ZmPHYA1和ZmPHYA2转录丰度能迅速响应黑暗到蓝光的转换(图4-B)。在0.25 h时ZmPHYA1急剧上升至自身黑暗中的15倍, 之后继续飙升, 在0.5 h时达到峰值(约为自身黑暗中的56倍); 随后其转录水平迅速下降, 在2 h时已基本恢复至自身黑暗的水平; 在4 h出现第2个峰值(自身黑暗中的5倍, 最大峰值的9%); 随后其转录丰度稳定在相对较低的水平(自身黑暗中的44.6%~71.7%)。同ZmPHYA1类似ZmPHYA2峰值也出现在0.5 h (约为黑暗中ZmPHYA1的10倍); 随后迅速下降, 在1 h后稳定在黑暗中ZmPHYA1的6.7%~23.7%的水平。可以看出, 二者均能迅速响应黑暗到蓝光的转换, 且光转换早期表达丰度上升快; ZmPHYA2的转录丰度在24 h内只有一个峰值; 而ZmPHYA1出现了两个峰值, 并且峰值极大(自身黑暗中的56倍)。以上结果表明ZmPHYA1和ZmPHYA2在蓝光信号途径中起重要作用, 而ZmPHYA1在对蓝光光质转换的响应更为重要。
ZmPHYA1和ZmPHYA2对黑暗到红光转换的转录表达模式基本一致(图4-C)。在转入红光0.25 h, ZmPHYA1的转录水平未发生变化; 在0.5 h达到第1个峰(黑暗中自身的3.4倍), 随后迅速下降, 在2 h恢复至自身黑暗起点时的水平, 而在4 h略有上升; 在8~12 h期间其转录丰度为自身黑暗的35%左右; 之后迅速上升, 在24 h已上升至转入红光24 h内的峰值(自身黑暗的4.5倍)。ZmPHYA2的转录模式整体与ZmPHYA1一致, 稍有不同的是在刚转入红光0.25 h内, ZmPHYA2的转录丰度先下降至ZmPHYA1黑暗的31%, 之后在0.5 h上升至第1个高于此时ZmPHYA1的峰值; 以后其转录丰度均低于ZmPHYA1, 在24 h, ZmPHYA2仅恢复至自身黑暗时的水平。由此看来, ZmPHYA1和ZmPHYA2在黑暗到红光转换的转录模式基本一致, 但从表达丰度上看, ZmPHYA1在前期作用更重要。
从图4-D可以看出, ZmPHYA1和ZmPHYA2由黑暗到白光转换的转录模式基本一致, 二者均在0.5 h时达到各自唯一的峰值, 此时ZmPHYA1和ZmPHYA2的转录丰度分别达到黑暗中ZmPHYA1的35和10倍, 之后迅速下降至低于黑暗水平并保持稳定。以上结果表明, ZmPHYA1和ZmPHYA2均迅速响应黑暗到白光的转换。
 | 图4 黑暗到不同光质转换条件下ZmPHYA1和ZmPHYA2转录表达的定量RT-PCR分析Fig. 4 qRT-PCR assays ofZmPHYA1 andZmPHYA2 expressions during transition from the dark to different light conditions |
玉米自交系B73幼苗在黑暗(Dk)生长13 d后转入远红光(FR, 1.9 µ mol m-2 s-1)(A)、蓝光(B, 13 µ mol m-2 s-1)(B)、红光(R, 22.3 µ mol m-2 s-1)(C)和白光(WL, 17 µ mol m-2 s-1)(D)下0、0.25、0.5、1、2、4、8、12和24 h的ZmPHYA1和ZmPHYA2表达模式。把ZmPHYA1在黑暗条件下的表达丰度设为对照, 并且将该ZmPHYA1/Tubulin设为1。折线图显示3次独立的生物学重复ZmPHYA/Tubulin的比值的平均值, 误差线代表标准差。
The seedlings of maize inbred line B73 were grown in the dark (Dk) for 13 d, then transferred to far-red light (FR, 1.9 µ mol m-2 s-1) (A), blue light (B, 13.0 µ mol m-2 s-1) (B), red light (R, 22.3 µ mol m-2 s-1) (C) and white light(WL, 17.0 µ mol m-2 s-1) (D) for 0.25, 0.5, 1, 2, 4, 8, 12, or 24 h. The transcription abundance ofZmPHYA1in the dark was set as the control, and the ratio of ZmPHYA1/Tubulin in the dark was set as 1. Each line chart shows the mean relative expression of ZmPHYA/Tubulin from three biological repeats. Error bars indicate the standard
deviation.
长日照处理条件下, ZmPHYA1和ZmPHYA2的转录表达模式一致(图5-A), 但是整体上ZmPHYA2转录丰度仅为ZmPHYA1的22%~51%。在光照和黑暗阶段各出现一个峰值。在光照阶段峰值出现在8 h, 此时ZmPHYA1和ZmPHYA2的转录丰度分别是各自在黑暗阶段结束时的2.4倍和4.6倍。黑暗阶段的峰值出现在进入黑暗后4 h, 此时ZmPHYA1和ZmPHYA2的转录丰度分别是各自在黑暗阶段结束时的7倍和5倍。在白光到黑暗转换时ZmPHYA1和ZmPHYA2都能迅速上升至较高水平。
ZmPHYA1和ZmPHYA2的转录表达模式对短日照处理的响应亦相似(图5-B), ZmPHYA2转录丰度仅为ZmPHYA1的21%~53%。在光照阶段, 二者转录丰度基本保持稳定状态。在转入黑暗后二者开始波动性上升, 在转入黑暗10 h均出现第1个峰值, 此时ZmPHYA1和ZmPHYA2的转录丰度分别是各自在黑暗阶段结束时的6倍和9倍。随后迅速下降, 2 h后达到最低(恢复至各自在黑暗阶段结束时水平)。在转入黑暗14 h达到第2个峰值(分别是各自在黑暗阶段结束时的9倍和5倍)。可见, ZmPHYA1和ZmPHYA2对长日照和短日照处理响应的表达模式极其一致, 前者转录丰度总体上是后者的2~5倍。
 | 图5 长日照和短日照件下ZmPHYA1和ZmPHYA2转录表达的定量RT-PCR分析Fig. 5 qRT-PCR assays ofZmPHYA1 andZmPHYA2 expression under long day and short day conditions |
玉米自交系B73的幼苗在长日条件(LD, 16 h光照/8 h黑暗, 图中, WL代表白光, Dk代表黑暗) (A)或者短日条件(SD, 8 h光照/16 h黑暗) (B)生长13 d, 然后每隔2 h取样一次。WL密度为17.0 µ mol m-2 s-1。把ZmPHYA1在黑暗阶段结束时的表达丰度设为对照, 并且将该ZmPHYA1/Tubulin设为1。折线图显示3次独立的生物学重复ZmPHYA/Tubulin的比值的平均值, 误差线代表标准差。
The seedlings of maize inbred line B73 were grown in long-day condition (LD, 16-h-light /8-h-dark, WL indicates white light, Dk indicates dark) (A) or short-day conditions (SD, 8-h-light /16-h-dark) (B) for 13 d, and the WL stands for continuous white light (17 µ mol m-2 s-1) , then they were harvested every two hours. The transcription abundance ofZmPHYA1in the dark was set as the control, and the ratio of ZmPHYA1/Tubulin at the end of the dark period was set as 1. Each line chart shows the mean relative expression of ZmPHYA/Tubulin of three biological repeats. Error bars indicate the standard deviation.
本研究表明, ZmPHYA1和ZmPHYA2在玉米的叶片以及花丝中表达量较高, ZmPHYA1表达量约是ZmPHYA2的2~8倍, 且ZmPHYA1在各个器官中的表达丰度均显著高于ZmPHYA2(图1)。玉米中胚轴伸长是响应不同光质处理的, 红光和白光下中胚轴较远红光和蓝光下更短(图2)。ZmPHYA1和ZmPHYA2的转录表达均响应远红光、蓝光、红光和白光处理(图3), 在黑暗到远红光、蓝光、红光或白光转换下ZmPHYA1的转录丰度均高于ZmPHYA2 (图4)。另外, 二者在长日照和短日照处理下, ZmPHYA1转录丰度总体上是ZmPHYA2的2~5倍(图5), 推测ZmPHYA1和ZmPHYA2在响应光周期上可能是不同的, 也暗示着二者功能可能存在差异。
水稻、玉米、小麦、高粱、谷子等是世界范围的粮食作物, 人类对它们已经进行了5000~10 000年的人工选择。作物的开花期和株高, 以及产量均与其栽培季节、纬度、海拔相适应, 因此这些性状都受到特定光照(红光/远红光)的调节[43]。光是影响玉米生长发育的重要因素, 减少光照时间会造成玉米早花[44]。玉米在1.4~2.0亿年前发生的异源四倍体化造成玉米中光敏色素基因为双拷贝[45, 46, 47, 48, 49]。ZmPHYB1与ZmPHYB2功能上的差异主要表现在phyB1抑制红光下的中胚轴伸长, 而phyB2主要负责光周期介导的开花调控[50]。我们的研究表明, ZmPHYA1和ZmPHYA2的转录丰度对黑暗到各种光质的转换, 以及长日照和短日照处理的响应存在差异, 这是否反映二者在功能上也存在差异, 还待进一步研究。
光受体过量表达的转基因植株节间缩短和植株矮化, 暗示修饰光信号转导途径同样能有效地改良株高和株型。在双子叶植物番茄中过量表达单子叶燕麦的PHYA基因, 导致植株显著降低[51]。在烟草中转化水稻类型I光敏色素(PHYA)后, 转基因植株表现下胚轴变短, 并且在伸长期生长速率减缓, 造成下胚轴变短的原因是表皮细胞变短而不是细胞数量的减少[52]。水稻phyA参与抑制幼苗中胚轴伸长[53]。玉米elm1突变体表现幼苗中胚轴变长、成株株高增加[54]。单子叶植物玉米中胚轴(mesocotyl)在功能上类似于双子叶植物的下胚轴, 可作为光形态建成的指标[54]。本研究表明玉米中胚轴伸长是响应光质处理的, 这种响应是否与光敏色素活性相关还需要进一步探究。
在拟南芥中, 长日照条件下CRY2与phyA通过与生物钟基因相互作用可以加速开花[55]。短日照植物水稻phyB或phyC突变体在长日照条件下均表现早花, 而phyA突变体却对开花时间没有影响, 但是phyA突变体在phyB或phyC突变体背景下却出现早花现象[56]。研究表明phyA参与光周期调节[26], 在长日照条件下, 水稻的phyA通过促进开花抑制因子GRAIN NUMBER及降低开花诱导物EARLY HEADING DATE 1的活性来导致开花推迟[56]。由此可见, 光信号转导途径与植物的开花期诱导紧密相关, 通过修饰光信号转导途径改良植物开花期性状是行之有效的方法[10]。本研究还表明, ZmPHYA1和ZmPHYA2转录丰度均强烈地响应在长日照和短日照处理, 玉米光敏色素是否参与其开花调控, 以及分子机制值得进一步探讨。
ZmPHYA1和ZmPHYA2主要在叶片和花丝中表达; 玉米中胚轴伸长是响应不同光质处理的, 远红光和蓝光下中胚轴较红光和白光下更长; ZmPHYA1和ZmPHYA2转录丰度较强地响应远红光和蓝光, 并呈现不同转录表达模式, ZmPHYA1在远红光下更重要, 而ZmPHYA2在蓝光下更重要。ZmPHYA1和ZmPHYA2的转录能有效地响应各种光处理, 可能ZmPHYA1在作物改良上比ZmPHYA2更有效。
The authors have declared that no competing interests exist.
作者已声明无竞争性利益关系。The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|


