第一作者联系方式: E-mail:xiaochun.w@163.com, Tel: 13783586761
谷氨酰胺合成酶(GS)是作物氮同化及转移利用的关键酶, 本试验研究了玉米灌浆期不同组织器官的GS同工酶表达特性, 鉴定了玉米GS同工酶的聚合方式。Western blot结果表明, 玉米不同组织器官的GS同工酶亚基表达存在明显差异, 分子量约40 kD的GS1亚基在所有组织中均大量表达, 39 kD的GS1亚基仅在穗位节及穗柄中大量表达, 分子量约44 kD的GS2亚基在叶片等光合组织中微量表达。通过改进BNE技术, 结合胶内转移酶活性的测定, 分析了玉米GS同工酶全酶的大小; 利用2-D胶结合Western blot鉴定了GS同工酶相应的亚基组成。结果表明, 在玉米组织鉴定出3种分子量不同的GS同工酶, GS2全酶分子量约460 kD, 为十聚体; GS1全酶有2种聚合状态, 一种是分子量约410 kD的十聚体, 另一种是分子量约240 kD的五聚体形式, 可见玉米GS同工酶表达存在多种方式。
Glutamine synthetase (GS) is a key enzyme in nitrogen assimilation and recycling in cereals. In this study, the expression characteristics of GS isoenzymes in different tissues and organs of maize in grain-filling period were analyzed, and the assembly of GS isoenzymes were indentified. The GS isoforms expressed differentially in different organs were shown by Western-blot obviously; GS1 with a molecular weight of about 40 kD expressed highly in all tissues, and GS1 with a molecular weight of about 39 kD was merely expressed in the node of ear position and pedical, and GS2 with a molecular weight of about 44 kD was weakly expressed in the photosynthtic tissue such as leaf. With a modified blue naive PAGE (BNE) technique and in-gel activity analysis, the size of GS holoenzyme was calibrated; combined the 2-D gel with western-blot analysis, the subunits composition of GS isoenzymes were identified. Three GS isoenzymes with different sizes were identified in maize. GS2 holoenzyme was about 460 kD and likely a decamer, GS1 holoenzyme existed two kinds of assembly state, one was about 410 kD and likely a decamer, another was about 240 kD and more likely a pentamer; therefore, the expression of GS isoenzymes exists diversity in maize.
氮是玉米生长发育必须的大量矿质营养元素, 也是玉米产量的一个主要限制因素。在高等植物中, 谷氨酰胺合成酶(GS)/谷氨酸合酶(GOGAT)循环是氮素同化的主要途径, 是无机氮转化为有机氮的枢纽[1], GS是GS/GOGAT循环中的关键酶, 因此, GS同工酶表达成为提高作物氮素利用率的一个研究热点。
高等植物中有2种GS同工酶, 定位于细胞液的GS1和质体的GS2。研究表明GS同工酶的表达受组织器官、生长发育、新陈代谢及环境因素等的调控[2, 3, 4]。GS1主要参与蛋白质等含氮有机化合物降解产生氨的再同化及转移利用[1], GS2主要参与光呼吸和硝酸盐还原产生的氨的同化[5]。高等植物GS2亚基较大(42~45 kD), 由单一核基因编码, 而GS1亚基较小(38~40 kD), 由2~5个核基因编码[6, 7, 8, 9, 10, 11, 12]。玉米GS2亚基约44 kD, 由单一核基因编码, GS1亚基39~40 kD, 由5个核基因编码[13]。
植物GS必须组装成聚合体才具有催化活性, 早期电子显微镜研究表明大豆GS1全酶是八聚体, 由2个平面环组成, 每个平面环由4个亚基组成[14]。Llorca等[10]利用X-ray晶体技术研究菜豆重组GS1结构, 发现其与大豆GS1全酶结构相同, 即由两个4元环聚合体组成的八聚体; 用分析离心机测定菜豆GS1全酶相对分子质量为344 kD, 与晶体研究结果一致。X-ray晶体分析表明玉米GS1、苜蓿GS1全酶是由2个五元环聚合体组成的十聚体[15, 16, 17], 但没有关于GS2全酶结构的报道。
利用凝胶过滤及分析离心机测定纯化GS全酶的分子量, 也可以初步判断GS的聚合状态, 但是所用材料多, 费时长, 仪器设备昂贵, 而且分辨率低。近年来Blue native PAGE (BNE)快速发展[18, 19], 利用凝胶电泳对蛋白质复合体依据其大小进行分离, 具有样品需求少、分辨率高、简便快捷且保持蛋白质聚合状态等特点, 越来越多地应用于活性蛋白的低聚物状态研究[20, 21, 22]。本研究通过改良BNE, 结合蛋白质免疫印迹等技术, 分析了玉米不同组织部位GS的表达特性, 并快速鉴定了GS同工酶的聚合状态, 与玉米GS1晶体研究结果一致, 为简便快捷及时研究GS同工酶表达调控方式及其与玉米氮素利用的关系提供了技术保障。
将玉米品种豫单916种植于河南农业大学农场(郑州), 常规田间管理, 于灌浆期选取根(地下节根和地上气生根)及不同叶位的叶片、叶脉、叶鞘、节、节间、穗柄、苞叶和籽粒, 快速清洗、剪碎, 于液氮中速冻, 置-80℃保存备用。
称取0.5 g不同组织部位的样品, 加液氮研磨, 再加3倍体积的提取缓冲液(100 mmol L-1 Tris-HCl, pH 7.6, 1 mmol L-1 EDTA, 1 mmol L-1 MgCl2和10 mmol L-1 β -巯基乙醇[13, 22])混成匀浆, 冰浴静置30 min后, 4℃、13 000× g离心30 min, 上清液即粗酶提取液。
利用3种凝胶电泳系统分离鉴定GS同工酶。
1.3.1 不连续活性聚丙烯酰胺凝胶电泳系统(Native- PAGE) 由3%的浓缩胶(pH 6.7)和5%的分离胶(pH 8.7)组成, 用于玉米不同组织器官的蛋白提取液中GS同工酶亚型的分离和酶活性检测[22]。4℃预冷电极缓冲液(25 mmol L-1 Tris, 192 mmol L-1甘氨酸), 粗酶液与5× 上样缓冲液[25 mmol L-1 Tris-HCl, pH 7.6, 5% (w/v) β -巯基乙醇, 0.05% (w/v)溴酚蓝, 50% (v/v)甘油]混匀后上样, 于4° C条件下电泳, 浓缩胶稳压80V, 分离胶稳压120 V。
1.3.2 Blue native PAGE (BNE) 依据Wittig等[18]改良BNE电泳方案, 根据GS同工酶全酶大小进行分离并鉴定其相对分子质量。凝胶由3%的浓缩胶和4%~13%的梯度分离胶组成, 样品处理同Native-PAGE, 加入预冷阳极缓冲液(25 mmol L-1咪唑-HCl, pH 7.0), 首先使用阴极电泳缓冲液A (0.02% Coomassie Blue G250, 50 mmol L-1 Tricine-HCl, 5 mmol L-1咪唑, pH 7.0), 于4° C、100 V电泳20min后, 更换为阴极电泳缓冲液B (50 mmol L-1 Tricine-HCl, 5 mmol L-1咪唑, pH 7.0), 继续电泳至样品进入4%~13%的梯度分离胶, 然后进行稳流(15 mA)电泳至蓝色指示剂出胶[22]。
1.3.3 Clear native PAGE (CNE) 参考Wittig等[19], 凝胶体系和BNE一样, 但只使用阴极电泳缓冲液B, 于100V电泳至样品进入分离胶, 然后进行稳流(15 mA)电泳至蓝色指示剂出胶。
胶内GS活性依据Wang等[22]方法检测, 活性染色结束后扫描结果, 然后再用考马斯亮蓝R250染色。BNE胶中, 以高分子蛋白Marker (Amersham HMW Calibration Kit For Native Electrophoresis)为标准, 利用Gel-Pro analyzer计算GS同工酶全酶的相对分子质量。
取适量粗酶提取液与等体积2× 的上样缓冲液[100 mmol L-1 Tris-HCl, pH 6.8, 4% (w/v) SDS, 10% (v/v) β -巯基乙醇, 0.2% (w/v)溴酚蓝, 20% (v/v)甘油]混合, 沸水浴5min变性处理, 室温条件下利用SDS-PAGE (5%浓缩胶, 12%分离胶)分离蛋白质, 将蛋白转移至PVDF膜上进行Western-blot检测。使用本实验室制备的小麦GS多克隆抗体检测玉米GS亚基, 使用Bio-Rad Clarity Western ECL试剂盒显色, 以蛋白Marker (Thermo Scientific PageRuler Prestained Protein Ladder)为标准, 利用Gel-Pro analyzer计算GS同工酶亚基分子质量。
切取BNE胶条, 浸泡于含1% SDS和1% β -巯基乙醇的变性液中, 37℃变性处理2 h[18], 然后用去离子水冲洗凝胶3~5次, 置SDS-PAGE浓缩胶顶部, 室温条件下电泳。电泳结束后, 将蛋白质转移至PVDF膜上进行Western blot分析。
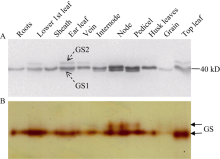
Western blot结果显示玉米中有3种GS亚基, 即40 kD和39 kD的胞液型GS (GS1)及44 kD的质体型GS (GS2), 且不同组织器官中GS同工酶表达差异较大(图1-A)。40 kD的GS1在玉米中起主导作用, 不同组织器官均有较大的表达量(籽粒除外); 39 kD的GS1仅在穗位节间、节和果穗柄中表达, 且后2个部位表达量较高, 可能与玉米灌浆期氮素营养的运输有关; GS2只在功能叶的叶片、叶脉中少量表达, 可能与玉米是C4植物, 光呼吸强度低有关。Native-PAGE结合胶内GS酶活性分析显示(图1-B), 玉米根系、叶片、茎节、穗柄等组织中均检测到GS活性带, 叶片中最高, 籽粒中最低; 但叶片提取液中仅检测到1条GS活性带, 可能因GS2活性太低或不连续Native-PAGE分离蛋白质的特点导致的2种GS同工酶没有被分离开; 此外与Western blot结果一致, 在含两种GS1亚基的穗位节和果穗柄中检测到2个GS同工酶。
通过改良BNE方法, 在玉米叶片和穗位节间、节和果穗柄中检测到2个大小不同的GS活性带, 其他组织中则只检测到一条GS带(图2-A)。其中, 迁移率较大的GS同工酶全酶分子量约240 kD, 活性较低; 迁移率较小的GS同工酶分子量约410 kD, 活性非常高。利用BNE分离玉米叶片总蛋白后, 进行Western blot, 检测到一个相对分子质量约460 kD的GS同工酶(图2-C), 可能因其活性低, 且分子量接近RuBP羧化酶, 其活性被羧化酶的蓝色条带遮盖[22]。
第一相胶为BNE, 用以分离玉米组织蛋白复合体, 切取相应组织的泳道进行蛋白质变性处理; 第二相胶为SDS-PAGE, 对BNE上的蛋白质复合体的亚基进行电泳分离; 然后转膜, 利用Western blot鉴定玉米组织中GS同工酶的亚基组成。玉米叶片中分子量约460 kD的GS同工酶(图2-A)由44 kD的亚基组成且信号非常弱(图3-A), 为GS2; 分子量约410 kD和240 kD的GS同工酶由39~40 kD的亚基组成且信号非常强, 为GS1, 表明胞液型GS存在两种不同的聚合方式。玉米穗柄中只有分子量约410 kD和240 kD的GS同工酶, 也是由39~40 kD的亚基组成, 说明穗柄中GS1全酶也存在两种不同的聚合方式。
利用BNE计算GS同工酶的全酶大小(图2), 利用BNE与SDS-PAGE结合进行两相电泳分离GS同工酶亚基, 利用Western blot检测鉴定GS同工酶亚基组成(图3)。利用GS同工酶分子量除以相应亚基的分子量, 在此基础上计算GS同工酶聚合状态, GS2为十聚体, 胞液型GS1有2种聚合方式, 一种为十聚体, 和前人结果一致[15]; 此外, GS1同工酶还存在另外一种聚合状态, 即五聚体。
高等植物2种GS同工酶分别定位于细胞液(GS1)和质体(GS2)[2, 23]。GS2缺失在正常条件下是致死性突变; 但通过抑制光呼吸, GS2突变体能够正常生长发育, 证明GS2参与光呼吸过程中的氨同化[2]。C3植物和C4植物光呼吸强度差异巨大[24], 小麦、水稻等C3植物光呼吸强度很高, GS2表达量更丰富[25], 而玉米等C4植物光呼吸强度要小得多, 本研究结果显示仅在玉米叶片中检测到GS2少量表达, 远远低于GS1表达量, 与C4植物光呼吸弱的生理现象一致。
玉米GS1基因由5个核基因编码, 其表达因组织、叶龄及氮量而异, Gln1-3和Gln1-4在叶片表达量高[26], Gln1-3决定穗粒数, Gln1-4调控粒重[13]。本研究发现玉米的穗位节、节间和穗柄组织中有2种高表达GS1亚基, 其中39 kD的GS1亚基是组织特异表达。推测39 kD的GS1可能在玉米灌浆期氮素营养转运方面起着重要作用。
由于从植物组织分离纯化足量蛋白的难度非常大, 因而, 通常采用异源重组植物GS研究其结构和聚合方式[10, 15, 16, 17]。BNE/CNE电泳技术用于分离膜蛋白等蛋白质复合物、测定天然蛋白复合体的相对分子质量, 且具有极高的分辨率[18, 19]。近年来广泛运用于植物可溶性蛋白的分离鉴定[20, 21, 22]。本研究通过改良BNE电泳技术分离玉米可溶性蛋白, 结合胶内活性测定、2-D胶和Western blot技术, 首次发现玉米叶片有3种分子量不同的GS同工酶, GS2为十聚体, GS1主要是十聚体, 与Unno等[15]利用晶体学X-ray分析的玉米GS1a是由2个五聚环组成的十聚体的结果一致; 此外, GS1还存在少量的五聚体, 推测玉米GS1的聚合状态也是一种在氮素代谢中广泛存在的GS同工酶调控方式。
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|