第一作者联系方式: E-mail:wangning_4306202@aliyun.com, Tel: 0372-2562282
以国欣棉3号为材料, 研究200 mg L-1缩节胺(DPC)浸种12 h对棉花子叶苗根系活力的影响, 并从活性氧(ROS)代谢的角度揭示相关的生理机制。结果表明, DPC浸种显著增强了棉花幼苗的根系活力, 根尖部位氯化三苯基四氮唑(TTC)染色光密度为清水对照的1.3倍, TTC法测定的根系活力和呼吸速率分别较对照增加167%和90%, 非损伤微测技术(NMT)测定的K+净内流速率 (距根尖300 μm处)较对照提高36%。吖啶橙染色结果显示, DPC处理根尖伸长区的凋亡细胞数目较对照减少。此外, DPC处理使根系的过氧化氢酶(CAT)、抗坏血酸氧化酶(APX)和谷胱甘肽还原酶(GR)活力显著高于对照, 超氧化物歧化酶(SOD)活力则降低; H2O2含量和超氧阴离子(O2-)产生速率较对照分别降低56%和65%, H2O2原位染色结果也显示其根尖部分的褐色较对照明显减弱。根系组织的ROS代谢得到改善可能是DPC浸种提高棉花幼苗根系活力的机制之一。
The plant growth regulator, mepiquat chloride (1,1-dimethylpiperidinium chloride, DPC) has been used worldwide to suppress excessive growth in cotton plants. It also increases the root vigour of cotton plants. To reveal the possible role of reactive oxygen species (ROS) involved, we conducted a experiment to investigate the effect of soaking seed with 200 mg L-1 mepiquat chloride (DPC) on root vigour of cotton (
缩节胺(DPC)是一种植物生长延缓剂, 化学名称为1,1-二甲基哌啶翁氯化物(1,1-dimethyl piperidium chloride), 它可阻断赤霉素的生物合成, 自20世纪80年代以来在中国、美国等主要植棉国家广泛用于控制棉花徒长[ 1, 2, 3, 4]。除了控制徒长, DPC还可促进棉花根系和产量器官的发育[ 5, 6, 7, 8, 9, 10], 并能增强根系活力、提高根系功能[ 2, 5, 11, 12]。
根系活力主要反映根系生命活动的旺盛程度和吸收、合成等功能的强弱程度。已有报道指出, DPC处理可以提高棉株根系合成氨基酸的能力[ 2], 增加伤流液的溢泌量[ 13], 增强对氮、磷、钾等矿质养分的吸收[ 2, 5, 10, 14]。但迄今为止, 尚不清楚DPC提高棉花根系活力的生理机制。活性氧(ROS)是一类具有氧化能力的分子、离子和自由基, 在植物细胞正常代谢过程中可通过多条途径产生[ 15]。ROS一方面可作为信号分子参与植物生长发育、细胞程序化死亡(PCD)和生物及非生物胁迫应答等生理过程, 另一方面作为强氧化剂具有极高的活性和毒性, 会引起一些大分子物质(如脂质、蛋白质和DNA等)的氧化破坏, 甚至引起细胞的死亡[ 16, 17]。有研究发现, DPC处理可以有效改善植株体内的ROS代谢、降低膜脂过氧化作用, 从而提高植株的抗旱和耐盐能力[ 18, 19]、延缓叶片的衰老[ 20]。为此我们推测, DPC处理提高棉花根系活力可能与改善根系的ROS代谢有关。
本文的研究目的, 一是采用氯化三苯基四氮唑(TTC)染色、吖啶橙染色和非损伤微测(NMT)等方法进一步验证DPC浸种提高棉花根系活力的作用, 二是探究根系组织中ROS及活性氧清除酶的变化, 试图从ROS代谢的角度揭示DPC浸种提高棉花根系活力的作用机制。
国欣棉3号由河北国欣种业有限公司培育并提供; DPC有效成分含量为97.5%, 由河北国欣诺农生物技术有限公司生产并提供。选取均匀饱满的种子分别置于清水(对照)和200 mg L-1的DPC溶液中, 于28℃下浸泡12 h, 然后用清水将种子冲洗干净, 于去离子水冲洗过的细河沙中萌发。培养室光照/黑暗时长为12 h/12 h, 温度为(30±2)℃/(20±2)℃。萌发5 d后子叶展开, 小心取出均匀一致的幼苗进行测定。
根系活力用氯化三苯基四氮唑(TTC)比色法测定根系的还原能力[ 21]。
根尖TTC染色及观察, 用双面刀片切下2~3 cm长的根尖, 迅速浸入含0.6%TTC的磷酸缓冲液 (50 mmol L-1, pH 7.4), 在30℃下避光染色24 h, 然后将样品转入55℃的95%酒精温育2 h, 取出吸干水分后在显微镜(BH-2, Olympus, Tokyo)下观察并照相。
K+的净流速测定, 在旭月(北京)科技有限公司采用非损伤微测技术(NMT, BIO-001B, Younger USA Sci. & Tech. Corp., Amherst, MA, USA)测定整株幼苗根尖部位的K+流速。用蒸馏水冲洗干净幼苗根系并在其中浸泡15 min, 转入20 mL测试液中平衡10 min, 再转入新的20 mL测试液中开始测试。测试液配方为0.1 mmol L-1KCl、0.1 mmol L-1CaCl2、 0.3 mmol L-1MES, pH 6.0; 为避免引入Na+和K+, 用低浓度Tris和HCl调节测试液pH值。每处理测定4株幼苗, 测试部位距根尖300 μm, 即根系分生区; 测试持续7~10 min, 计算时舍弃前2~3 min的数据[ 22]。
吖啶橙染色参考Ünal和Uyanikgil[ 23]的方法, 将离体幼苗根系插入含有0.05 mg mL-1吖啶橙的磷酸缓冲液(10 mmol L-1, pH 7.0), 在室温下避光染色30 min, 然后取出用10 mmol L-1磷酸缓冲液冲洗3次, 吸干水分后在激光共聚焦显微镜(SP5, Leica, Germany)下观察并照相。
呼吸速率应用红外线CO2分析仪(CIRAS-2 portable IRGA system, PP-systems, Amesbury, MA, USA)测定[ 24]。
相对电导率参考Nayyar等[ 25]的方法, 应用电导仪(EC 215, Hanna Instruments, USA)测定。
抗氧化酶活性及其同工酶染色参照Gossett等[ 26]的方法, 酶提取液为50 mmol L-1磷酸缓冲液[pH 7.0, 含1% (w/v) PVP、0.1 mmol L-1 EDTA-Na2和0.05% (w/v) Triton X-100]。取0.5 g新鲜的幼苗根尖(2~3 cm长), 冰浴条件下研磨成匀浆, 然后在4℃下12 000× g离心15 min, 取上清液待测。超氧化物歧化酶(SOD, EC 1.15.1.1)、过氧化物酶(POD, EC 1.11.1.7)、过氧化氢酶(CAT, EC 1.11.1.6)、抗坏血酸氧化酶 (APX, EC 1.11.1.11)和谷胱甘肽还原酶(GR, EC 1.6.4.2)等抗氧化酶活性的测定、同工酶分离和染色均采用Parida等[ 27]的方法。
H2O2活体组织原位检测参照Romreo-Puertas等[ 28]的方法, 将离体幼苗根系插入含1% 3,3-二氨基联苯胺(DAB)的磷酸缓冲液(10 mmol L-1, pH 6.5), 室温下避光吸收8 h, 取出吸干水分后在显微镜(BH-2, Olympus, Tokyo)下观察并照相。
H2O2含量和超氧阴离子(O2-)生成速率, 取0.5 g新鲜幼苗根尖(2~3 cm长), 在冰浴中用100 mmol L-1磷酸缓冲液(pH 7.8, 含1% PVP)研磨成匀浆, 于4℃下12 000× g离心20 min, 取上清液测定H2O2含量和O2-生成速率[ 29]。
所有试验至少独立重复3次, 各次结果趋势一致, 取其中具有代表性的数值进行统计分析。用SPASS 16.0 (SPSS Inc. Chicago, USA)的 t检验对平均值进行比较( P<0.05)。
TTC是一种水溶性物质, 通过细胞膜进入细胞内被脱氢辅酶(NADH或NADPH)还原变成红色, 常用来表征根系活力。从图1可知, DPC浸种根系的TTC染色较对照深, 单位面积光密度为对照的1.3倍( P<0.05)。此外, 以TTC法测定的根系活力为108.4 µg g-1 h-1, 较对照的40.6 µg g-1 h-1提高了167% ( P<0.05)。
 | 图1 DPC浸种处理对棉花幼苗根系活力(TTC染色)的影响Fig. 1 Effect of soaking seeds with DPC on root vigour of cotton seedlings determined by triphenyl tetrazolium chloride (TTC) staining (×500 μm) |
DPC浸种处理使棉花幼苗根尖部位(2~3 cm)的呼吸速率显著提高90% (图2-A), 表明根系生命活动比较旺盛。根系分生区的吸收能力也得以增强, K+净内流速率由对照的-292.85 pmol cm-2 s-1显著增加到 -399.30 pmol cm-2 s-1(负值表示内流, 即吸收; 图2-B), 提高幅度为36% ( P<0.05)。此外, DPC浸种处理根尖部位(2~3 cm)的相对电导率由对照的64%降低到56%, 提示其根系细胞膜完整性好、透性低。
 | 图2 DPC浸种处理对棉花幼苗根系呼吸速率(A)和K+净内流速率(B)的影响Fig. 2 Effect of soaking seeds with DPC on root respiration rate (A) and net K+ fluxes in root meristematic zone (B) of cotton seedlings |
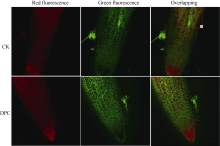
吖啶橙具有膜通透性, 可使细胞中的DNA和RNA染色, 与DNA结合时细胞核发出均匀的绿色或黄绿色荧光, 与RNA结合时细胞质发出均匀的桔黄色或桔红色荧光。在凋亡的细胞中, 染色质固缩或断裂为大小不等的片段, 吖啶橙会使其染上致密浓染的绿色荧光。比较图3可知, DPC处理根系细胞的桔红色着色较对照均匀, 荧光亮度也比较高(光密度为对照的2.3倍), 表明其细胞状态正常、细胞活力较高。从图3中可见, 对照根系伸长区的部分细胞中出现致密浓染的绿色荧光斑点(a区), 提示该区发生了细胞凋亡, 而DPC浸种根系的相应区域未发现类似现象。本试验中根系细胞胞壁的绿色荧光比较强, 是因为其中的木质素也可被吖啶橙染成绿色[ 30, 31]。
DPC浸种处理的根系SOD活力较对照显著降低22% (表1), 7条同工酶带的表达量也降低(图4), 其中自上而下第2、第6、第7条酶带的光密度分别为对照的71%、53%和70%。与之相反, 浸种处理的CAT、APX和GR活力分别较对照显著增加32%、67%和51%, CAT和APX同工酶带的光密度也分别较对照提高90%和70% (图4), GR自上而下第1、第4、第5条同工酶带的光密度则分别较对照提高40%、80%和50% (图4)。DPC浸种处理对POD活力和同工酶表达量均无显著影响(表1和图4)。
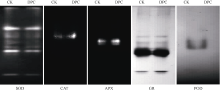 | 图4 DPC浸种处理对棉花幼苗根系抗氧化酶同工酶表达量的影响Fig. 4 Effect of soaking seeds with DPC on ROS isoenzymes in roots of cotton seedlings |
| 表1 DPC浸种处理对棉花幼苗根系ROS活力的影响 Table 1 Effect of soaking seeds with DPC on ROS activity in roots of cotton seedlings (U mg-1 Protein) |
DAB是过氧化物酶的生色底物, 在H2O2存在下失去电子而形成褐色沉淀。由图5可见, DPC浸种处理根系染色较浅且染色面积较小, 染色光密度仅为对照的23%。化学测定结果也显示DPC浸种处理使根系中的H2O2含量较对照降低了56% (图6)。此外, 处理根系的O2-产生速率也较对照降低65% (图6)。
 | 图5 DPC浸种处理对棉花幼苗根系H2O2活体组织原位染色的影响Fig. 5 Effect of soaking seeds with DPC on H2O2 histochemical detection in situ by diaminobenzidine staining in roots of cotton seedlings (×500 μm) |
根系活力泛指根系的吸收、合成及氧化和还原能力。本文结果表明, DPC浸种处理根系的还原能力(TTC法)增强(图1), 呼吸速率提高(图2-A), 凋亡细胞减少(图3), K+净内流速率提高(图2-B), 这为DPC处理增强棉花根系活力提供了更多的证据。
本文还发现, DPC浸种处理显著提高了CAT、APX和GR的活力及同工酶表达量, 但降低了SOD的活力及其同工酶表达量。已有研究表明, SOD催化植物组织中的O2-生成H2O2, 而CAT、APX和GR可将H2O2进一步分解为无毒害作用的H2O和O2[ 15]。作者推测, DPC浸种处理既可减少幼苗根系H2O2的生成, 又可加速H2O2的降解, 因此显著降低了根系中H2O2的含量(图6)及其DAB染色强度(图5)。也有报道指出, DPC影响植株体内活性氧清除酶的活性, 可以促进ROS的清除、维护细胞膜的稳定、延缓叶片的衰老、提高植株的耐盐能力等[ 19, 20]。O2-的产生源于电子传递链运行时电子的漏出, 漏出的电子直接与O2进行单电子还原形成O2-, 对细胞具有高毒性[ 32, 33]。本研究中, DPC浸种处理根系的O2-产生速率显著低于对照(图6), 可能是因为处理根系较高的呼吸速率(图2-A)和/或较强的还原能力(图1)减少了电子传递链的电子漏出。
以往研究证明, ROS能改变细胞内相对稳定的氧化还原状态[ 34], UV-B辐射诱导的ROS积累可以抑制NADPH硝酸还原酶的活性[ 35]。Desikan等[ 36]报道, 外源H2O2可以激发拟南芥悬浮细胞中与细胞程序化死亡(PCD)有关的mRNA和蛋白质合成, H2O2浓度越高, 细胞死亡的速度越快。Storey和Walker[ 37]和马怀宇等[ 38]的研究也表明, 胁迫条件下果树产生大量ROS, 从而诱导根系细胞死亡, 加速根尖木栓化。此外, ROS能激活膜上的非选择性阳离子通道和部分外排的离子通道, 促进K+的外排、降低根系细胞的净吸收能力[ 39, 40]。因此作者推测, 本试验中DPC浸种处理提高棉花幼苗根系的还原能力、减少凋亡细胞数目、促进根系K+净吸收能力与改善ROS代谢、降低ROS的积累有密切关系。
经DPC浸种处理的棉花幼苗根系活力增强, 表现为根系还原能力(TTC法)和呼吸速率提高、细胞膜透性降低、凋亡细胞减少、K+净内流速率提高。DPC浸种处理还显著改善了棉花幼苗根系的ROS代谢水平, SOD活力降低, CAT、APX和GR等酶活力增强, 均降低了根系中H2O2的积累; DPC浸种处理根系的O2-产生速率也显著低于对照。
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|




 −, superoxide radicals; OH
−, superoxide radicals; OH