聚合兼抗白粉病、条锈病和叶锈病的慢病性基因, 是培育持久多抗小麦品种的重要措施。百农64和鲁麦21均为慢白粉病品种, 分别含有4个和3个慢白粉病抗性QTL。将百农64与鲁麦21杂交, 获得21个聚合2~5个慢白粉病抗性QTL的F6株系, 于2012—2013年度分别在四川郫县和甘肃天水进行条锈病田间抗性鉴定,在河北保定和河南周口进行叶锈病田间抗性鉴定。分析21个株系条锈和叶锈病的最大严重度和病程曲线下面积, 检测单个QTL和QTL聚合体对条锈病和叶锈病的抗性效应。结果表明,
Pyramiding quantitative trait loci (QTLs) is an effective method to improve resistance to powdery mildew, stripe rust, and leaf rust in common wheat. We have developed 21 lines (F6) carrying 2-5 slow mildewing QTLs by crossing slow powdery mildew cultivars Bainong 64 and Lumai 21 possessing four and three slow mildewing QTLs, respectively. These F6lines were evaluated in the field in Pianxian, Sichuan and Tianshui, Gansu for stripe rust resistance and in Baoding, Hebei and Zhoukou, Henan for leaf rust resistance during the 2012-2013 cropping season. According to the maximum disease severities (MDS) and the area under the disease progress curve (AUDPC), QTLs
小麦白粉病、条锈病和叶锈病是全球性重要病害, 分别由小麦白粉菌( Blumeria graminisf. sp. tritici)、小麦条锈菌( Puccinia striiformis f. sp. tritici)和小麦叶锈菌( P. recondita f. sp. tritici)引起, 具有发生频率高、流行范围广和暴发性强的特点, 可致3.0%~49.0%的产量损失[ 1, 2, 3]。长期以来, 利用寄主抗性防治这些病害取得了显著成效[ 4]。
寄主抗性分为垂直抗性和水平抗性。垂直抗性又称为生理小种专化性抗性、苗期抗性、全生育期抗性或主效基因抗性, 是由1个或少数几个主效基因控制, 对特定病原菌生理小种表现出高抗或免疫, 具有病原菌生理小种专化性, 常因病原菌生理小种变异而丧失抗性; 水平抗性亦称慢病性、成株抗性或非小种专化抗性, 由多个微效基因控制, 对病原菌无小种专化性或专化性弱, 苗期表现为感病, 成株期表现为中抗或高抗, 且抗性持久[ 5, 6]。慢病性由多个微效基因的加性效应控制, 基因聚合是获得慢病性、选育兼抗多种病害品种的重要方式。Singh等[ 7]发现, 聚合4~5个微效慢病基因的小麦材料对条锈和叶锈病呈高抗至免疫。意大利小麦品种Strampelli和Libellula及我国农家种平原50至今仍然高抗条锈病。抗性遗传分析表明, Strampelli和Libellula含有 Yr18/Lr34/Pm38/Sr57和2~4个其他慢病基因[ 8]; 平原50含有3个慢条锈基因和3个慢白粉基因[ 9]。 Yr18/Lr34/Pm38/Sr57对小麦条锈病、叶锈病、白粉病和秆锈病均具有抗性[ 10, 11]。
随着QTL定位和分子标记研究工作的不断深入, 育种家可以利用分子标记聚合抗病QTL, 培育持久抗性品种[ 7, 12, 13]。从20世纪70年代开始, 国际玉米小麦改良中心(CIMMYT)选育出一批抗性持久且兼抗多种病害的慢病性品种, 例如Amadina、Chapio、Cook、Kukuna、Parula、Pavon 76、Sonoita 81、Tonichi 81和Tukuru, 均含有 Yr18/Lr34/Pm38/Sr57或 Yr29/ Lr46/Pm39/Sr58位点及2~3个微效基因[ 14, 15]。因此, 聚合慢病性基因是获得兼抗多种病害的持久抗性小麦品种的重要手段。
虽然小麦抗病性QTL定位研究很多, 但是由于不同遗传背景下的QTL聚合到同一遗传背景存在诸多困难[ 16], 不同来源的QTL可能存在互作, QTL聚合育种实践的报道很少。Miedaner等[ 17]将3个来源不同的赤霉病抗性QTL聚合到同一种质, 发现单个QTL均可降低赤霉病发病率和脱氧雪腐镰刀菌烯醇(DON)含量, 但3A上的一个QTL单独存在或与3B和5A上QTL聚合时对赤霉病没有显著遗传效应。Lu等[ 18]将5A上的QTL与 Fhb1聚合在一起, 发现可以降低矮秆基因 Rht-D1b对赤霉病引起的效应。可见, QTL聚合体遗传效应分析具有重要的应用价值。
百农64和鲁麦21均具有优良的农艺性状, 分别在20世纪90年代和21世纪初为我国黄淮麦区主推品种, 其苗期对白粉菌[ 19]、条锈菌和叶锈菌[ 20]流行小种均感病, 但田间成株期表现抗病, 具有典型的慢病性特征。在百农64中检测到4个慢白粉病抗性QTL ( QPm.caas-1A、 QPm.caas-4DL、 QPm.caas- 6BS和 QPm.caas-7A)[ 21], 在鲁麦21中检测到3个慢白粉病抗性QTL ( QPm.caas-2BS、 QPm.caas-2BL和 QPm.caas-2DL)[ 22]。Bai等[ 23]采用杂交和改良系谱法, 对百农64和鲁麦21所含慢白粉病抗性QTL进行聚合, 创制了21个F6聚合株系, 并对这些材料的白粉病抗性进行了分析。在此基础上, 本研究将分析这21个聚合不同慢白粉病基因的株系对条锈病和叶锈病的遗传效应, 明确这些慢白粉病抗性QTL及QTL聚合体对条锈病和叶锈病的抗性, 发掘高抗条锈、叶锈和白粉病的慢病性材料和QTL组合, 为选育兼抗多种病害的持久抗性小麦品种提供材料和方法。
百农64与鲁麦21杂交, 采用改良系谱法, 根据田间白粉病抗性和综合农艺性状选择, 在早代淘汰白粉病严重度高的株系, 选择低严重度株系继续种植, 经过连续多代鉴定和选择, 获得21个F6抗病株系(编号: BFB5~BFB25)[ 23]; 利用16个分子标记[ 21, 22]进行检测, 明确这些株系携带2~5个慢白粉病QTL。这些慢白粉病QTL中, QPm.caas-1A的连锁标记为 Xbarc148和 Xwmc550, QPm.caas-4DL的连锁标记为 Xgwm165、 Xcfd23和 Xwmc331, QPm.caas-6BS的连锁标记为 Xbarc79和 Xgwm518, QPm.caas-7A的连锁标记为 Xbarc127和 Xbarc174, QPm.caas-2BS的连锁为 Xbarc98和 Xbarc1147, QPm.caas2BL的连锁标记为 Xbarc1139和 Xgwm47, QPm.caas-2DL的连锁标记为 Xbarc18、 Xgwm539和 Xcfd233。
将21个F6聚合基因株系及其亲本于2012—2013年度种植于四川郫县(30°05′N, 102°54′E)和甘肃天水(34°05′N, 104°35′E)进行条锈病抗性鉴定; 在河北保定(113°40′N, 38°10′E)和河南周口(114°38′N, 33°37′E)进行叶锈病抗性鉴定。采用完全随机区组设计, 3次重复, 单行区, 行长1.50 m, 行距0.25 m, 每行种植50粒。在郫县和天水点, 每10行种植1行高感条锈病品种对照辉县红, 小区周围种植高感品种作为诱发行; 在保定和周口, 每10行种植1行高感叶锈病对照郑州5389。以保证充分接种, 实际观察所有对照均发病完全。
接种后6周左右, 当对照品种充分发病时开始调查发病严重度, 记录旗叶和倒二叶上条锈菌或叶锈菌孢子堆面积占总叶片面积的百分数。每隔7 d调查一次, 共调查2~3次, 直到叶片上孢子堆不再增加为止。
用最大严重度(maximum disease severity, MDS)和病程曲线下面积(area under the disease progress curve, AUDPC)作为抗病性评价指标。
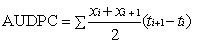
式中, xi表示第 i次调查的严重度, ti表示第 i次调查距接种后的天数[ 24]。用SAS 9.2软件进行统计分析和显著性比较。
MDS和AUDPC在株系间和环境间的差异均达极显著水平( P<0.01)。百农64和鲁麦21在郫县和天水两个环境下条锈病平均MDS分别为36.8%和50.0%, 平均AUDPC分别为210.6和290.5; 12个聚合基因株系的条锈病平均MDS均低于百农64和鲁麦21。其中, 株系BFB14在两个环境下的条锈病平均MDS值(16.2%)和平均AUDPC值(92.1)最低; 在保定和周口的叶锈病平均MDS分别为15.8%和35.0%, 平均AUDPC分别为91.6和212.3。其他11个株系的叶锈病平均MDS低于百农64和鲁麦21。株系BFB9在两个环境下叶锈病的平均MDS (3.6%)和平均AUDPC (18.9)最低(表1)。
| 表1 21个聚合基因株系及其亲本3种病害的最大严重度(MDS)和病程曲线下面积(AUDPC) Table 1 Composition of slow mildewing QTL, and averaged MDS and AUDPC for powdery mildew, stripe rust and leaf rust response in 21 F6 lines from the Bainong 64/Lumai 21 cross and their parents |
QPm.caas-4DL、 QPm.caas-2BS和 QPm.caas-2BL对条锈病具有显著的抗性遗传效应( P<0.01), 分别解释条锈病平均表型变异的16.9%、14.1%和17.3%, QPm.caas-6BS可解释1.8%的表型变异。 QPm.caas- 2BS和 QPm.caas-2BL之间存在互作, 但效应较低, 环境与QTL间也存在互作。 QPm.caas-4DL对叶锈病也具有显著抗性, 可解释表型变异的35.3%, 而 QPm.caas-6BS可解释8.3%的表型变异(表2)。
| 表2 4个环境下慢白粉病抗性QTL对条锈病和叶锈病的效应及其互作 Table 2 Effects of slow mildewing QTL on stripe rust and leaf rust, and their interactions from four environments |
百农64和鲁麦21以及21个慢病性QTL聚合株系中共有11种QTL组合(表3)。 QPm.caas-1A/ QPm.caas-4DL/ QPm.caas-2DL/ QPm.caas-2BS/ QPm.caas-2BL和 QPm.caas-1A/ QPm.caas-4DL/ QPm.caas- 2BS/ QPm.caas-2BL聚合体的条锈病MDS及AUDPC值最低, 远低于两亲本及其他7种QTL聚合体, 且差异显著( P<0.05), 对条锈和叶锈病有较好的抗性。 QPm.caas-1A/ QPm.caas-4DL/ QPm.caas-7A组合的条锈病MDS和AUDPC最高, 与鲁麦21差异不显著, 但显著高于百农64及其他8种QTL聚合体( P<0.05), 21个聚合株系中其余6种QTL聚合体抗性与百农64差异不显著。
| 表3 不同慢白粉病抗性QTL聚合体对白粉病、条锈病和叶锈病抗性效应 Table 3 Effects of different slow mildewing QTL combinations on powdery mildew, stripe rust, and leaf rust response |
QPm.caas-1A/ QPm.caas-4DL/ QPm.caas-2DL/ QPm.caas-2BS/ QPm.caas-2BL、 QPm.caas-1A/ QPm.caas- 4DL/ QPm.caas-2BS/ QPm.caas-2BL和 QPm.caas-1A/ QPm.Caas-4DL/ QPm.caas-2BS组合的叶锈病MDS及AUDPC值较低, 显著低于双亲及其他6种QTL聚合体, 且差异显著( P<0.05), 对叶锈病有较好抗性。 QPm.caas-1A/ QPm.caas-4DL/ QPm.caas-7A和 QPm.caas-4DL/ QPm.caas-2BS组合的叶锈病MDS和AUDPC较高, 与鲁麦21差异不显著, 但显著高于21个聚合株系中其他7种QTL聚合体( P<0.05), 其余4种QTL聚合体抗性与百农64无显著差异。
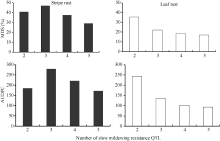
除株系BFB12外, 随着含有白粉病抗性QTL数量的增多, 聚合株系的条锈病和叶锈病MDS和AUDPC呈现逐渐降低趋势(图1)。
利用分子标记辅助选择聚合慢病性基因是培育兼抗白粉病、条锈病和叶锈病品种的重要途径。迄今为止, 已证实 Yr18/Lr34/Pm38/Sr57、 Yr29/Lr46/ Pm39/Sr58和 Yr46/Lr67/Pm46/Sr55等多个小麦慢病性基因兼抗条锈病、叶锈病、白粉病和秆锈病[ 26, 27, 28, 29]。同时, 还在1BL、2BS、2BL、3BS、6BS和7DS上发现了多个兼抗条锈病、叶锈病和白粉病的基因簇[ 30]。这些基因的发现为培育兼抗多种病害的小麦品种提供了可能。CIMMYT约60%的小麦品种聚合多个慢病性基因, 并已建立通过聚合 Yr18/Lr34/ Pm38/Sr57、 Yr29/Lr46/ Pm39/Sr58和 Yr46/Lr67/Pm46/ Sr55等几个慢病性基因, 选育兼抗几种病害的持久抗性品种的主要育种策略, 运用该策略成功选育出一批兼抗型小麦品种[ 4]。携带 Yr18/Lr34/Pm38/Sr57基因的材料在CIMMYT小麦种质资源中广泛存在, 已保持70多年的抗性, 发展中国家含 Yr18/Lr34/ Pm38/Sr57的小麦品种种植面积约有2600万公顷[ 31], 在病害流行年份发挥着重要作用。美国、澳大利亚和欧洲的研究重点近年也从垂直抗性逐步转向慢病性[ 32, 33, 34]。
百农64含有的抗白粉病 QPm.caas-4DL与 Yr46/ Lr67/Pm46/Sr55很可能为同一基因, 虽然 Yr46/Lr67/ Pm46/Sr55抗性效应不及 Yr18/Lr34/Pm38/Sr57, 但百农64的农艺性状良好, 该位点将是小麦持久抗病育种的另一重要基因资源。Ren等[ 25]在 QPm. caas-6BS同一位置分别发现条锈病和叶锈病抗性QTL, 该位点是一个新发现的兼抗多种病害的位点。任妍[ 20]在鲁麦21中定位了2个条锈病抗性QTL QYr.caas-2BS和 QYr.caas-2BL, 分别与抗白粉病 QPm.caas-2BS和 QPm.caas-2BL位置一致, 这2个位点也是小麦持久兼抗育种的重要基因。利用慢病性基因容易做到兼抗与持久抗性的结合, 兼抗几种病害的慢病基因都具有持久抗性(Ravi Singh, 个人交流)。已经定名的几个慢病性基因(如 Yr18/Lr34/ Pm38/Sr57、 Yr29/Lr46/Pm39/Sr58和 Yr46/Lr67/Pm46/ Sr55等)都具有这种特点。我国小麦白粉病、条锈病和叶锈病发生严重, 选育兼抗多种病害小麦品种对于我国小麦生产具有重要意义。来自百农64的 QPm.caas-4DL、 QPm.caas-6BS和来自鲁麦21的 QPm.caas-2BL和 QPm.caas-2BS等对条锈病、叶锈病和白粉病皆表现慢病性, 利用这些基因选育兼抗多种病害的小麦品种可取得事半功倍的效果。虽然过去国内也有育成兼抗且具有持久抗性的小麦品种, 但有目的地进行这种育种的尚不多。
在21个聚合株系中, 含有抗病QTL数量较多的株系的MDS和AUDPC值均较低, 表明QTL加性效应起了重要作用。Singh等[ 7]发现聚合4~5个微效基因的小麦材料对条锈和叶锈病呈高抗至免疫。但是, QTL聚合体的遗传效应并不总是与所含抗病QTL的数量成正比。如株系BFB12和BFB14分别含有2个和3个抗性QTL, 但条锈病MDS和AUDPC值均较低。其原因可能是不同的QTL聚合体效应不同, 部分QTL间互作会产生加性效应, 或者某些株系含有未知的抗性QTL, 导致条锈病和叶锈病MDS和AUDPC值较低, 抗性增强。
任妍[ 20]在鲁麦21中还发现另外2个条锈病抗性QTL ( QYr.caas-4DL.2和 QPm.caas-2DS.2), 我们推测本研究的部分株系可能含有这2个QTL, 导致BFB12和BFB14等含有较少兼抗型QTL的株系抗性较强。相同QTL聚合体的株系抗性并不完全一致, 如BFB11和BFB15均含有 QPm.caas-1A/ QPm. caas-4DL/ QPm.caas-2DL/ QPm.caas-2BS基因组合, 但BFB11抗性远大于BFB15, 估计BFB11含有其他条锈病抗性QTL或存在QTL间互作。但综合21个株系数据来看, 株系含有QTL越多, 对多种病害的抗性越强。
结合Bai等[ 23]对21个聚合株系的白粉病鉴定结果进行分析, 株系BFB5、BFB7、BFB8、BFB10、BFB11和BFB14对白粉病、条锈病和叶锈病均具有较高的抗性, 可作为育种亲本材料用于小麦抗锈病和白粉病育种。 QPm.caas-1A/ QPm.caas-4DL/ QPm. caas-2DL/ QPm.caas-2BS/QPm.caas-2BL、QPm.caas-1A/ QPm.caas-4DL/ QPm.caas-2DL和 QPm.caas-1A/ QPm. caas-4DL/ QPm.caas-2BS组合对白粉病、条锈病和叶锈病均具有较好的抗性, 是选育兼抗多种病害小麦慢病性品种的良好材料。 QPm.caas-1A/ QPm.caas- 4DL/ QPm.caas-2BS/QPm.caas-2BL对条锈病和叶锈病抗性较好, 可用于条锈病和叶锈病多发区的抗病育种。
6个聚合百农64和鲁麦21慢白粉病抗性QTL的F6株系对白粉病、条锈病和叶锈病均具有较好的慢病性, 且农艺性状优良, 是选育抗病高产品种的优良材料。证实了利用分子标记进行慢病性QTL聚合的可行性及其有效性, 进一步说明聚合4~5个慢病性QTL足以在田间表现高水平抗性, 为小麦抗病育种提供了材料和育种思路。
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|