第一作者联系方式: E-mail:mianbaohua2008@126.com
种子休眠性是花生重要的农艺性状, 外源乙烯利能诱导花生种子休眠的解除, 为了阐明乙烯利作用下花生种子休眠解除的分子机制, 设置吸胀的休眠种子为对照, 100 mg L-1乙烯利处理吸胀休眠种子后不同时间的样品(AE1、AE2、AE3)进行转录组分析, 比较了花生种子休眠解除过程中ABA、GA、ETH、auxin相关基因的表达。结果表明, 15个与GA、40个与ABA、60个与ETH、56个与auxin相关的unigenes在花生种子休眠解除过程中表现显著差异表达。荧光定量PCR结果显示, ABA合成关键基因 AhNCED2和代谢关键基因 AhCYP707A1在种子休眠解除过程中均受外源乙烯利诱导, 表达差异显著; 在休眠和无休眠种子吸胀萌发过程中, AhNCED2和 AhCYP707A1的表达趋势不同, AhNCED2对于种子休眠的维持发挥积极作用, 而 AhCYP707A1对于种子休眠解除发挥积极作用。
Seed dormancy is one of important agronomic traits in peanut ( Arachis hypogaea L.). Seed dormancy can be released with exogenous ethephon. To understand the molecular mechanisms of switches from dormancy to germination in peanut seeds underlying the role of ethephon, we preformed transcriptome analyses among imbibed dormant seeds as control and dormancy-released seeds (AE1, AE2, AE3) treated by 100 mg L-1exogenous ethephon, and compared the expression of unigenes related to ABA, GA, ETH and auxin. The results showed that there were 15, 40, 60, and 56 unigenes associated with GA, ABA, ETH, and auxin respectively, which were significantly differentially expressed unigenes during the process from dormancy to germination. The results of Real-time RT-PCR showed that the expressions of AhNCED2and AhCYP707A1 were induced distinctly by exogenous ethephon in seed dormancy released process. In dormant and non-dormant seed imbibition and germination processes, there were different roles between expresses of AhNCED2 and AhCYP707A1. AhNCED2played a positive role in maintaining seed dormancy, while AhCYP707A1played a positive role for seed dormancy breaking.
休眠和萌发过程中, 激素扮演着非常重要的角色。ABA对诱导和维持种子休眠有积极的调控作用, GA对终止种子休眠与促进发芽有着重要的作用。种子吸胀萌发过程中, ABA合成量增加和GA含量降低维持种子休眠, 反之促进种子萌发[1, 2]。休眠态种子吸胀时, ABA将重新开始合成, 使种子保持休眠态; 非休眠态种子吸胀时不会出现这种情况[3, 4]。Ali-Rachedi等[5]发现强休眠拟南芥Cvi种子胚中存在高含量ABA, 其含量随着种子的休眠解除而降低。Cadman等[6]在转录组水平证实种子休眠与ABA生物合成有关。特别是ABA生物合成关键酶NCED (9-cis-epoxycarotenoid dioxygenase)和ABA降解途径关键酶CYP707A[(+)-abscisic acid 8’ -hydroxylase] 对于种子休眠具有普遍意义[7]。通过对拟南芥ABA代谢缺陷型突变体的研究发现, 编码ABA 8’ 羟化酶的基因(CYP707A1、CYP707A2)失活会导致种子休眠性增强[8, 9]。种子发育过程中, 乙烯对ABA有拮抗作用, 拟南芥乙烯受体突变体etr1种子休眠性较野生型增强, 并且突变体干燥种子中ABA的浓度是野生型种子的8倍[13]。乙烯促进GA缺陷型突变体的萌发表明乙烯的合成参与了种子休眠的解除[14, 15], 同时乙烯与GA有协同作用, 促进胚生长伸长, 软化胚根周围组织, 是种子萌发所必需的[11, 12]。
花生是我国重要的油料作物和经济作物, 面积、单产、总产均居世界前列。近年来我国花生生产中多有报道[16, 17], 在花生收获时期恰逢阴雨天气导致花生在植株上发芽, 严重影响了花生的产量和品质, 其主要根源是目前选育推广的花生新品种休眠性较弱, 因此选育具有适度休眠性的花生新品种是行之有效的解决方法。目前有关花生种子休眠性 的研究在休眠性检测方法、影响因素、品种鉴选和遗传分析方面均有报道[18, 19, 20, 21, 22, 23, 24, 25, 26, 27], 而花生种子休眠以及解除休眠过程中的分子机制尚未见报道。本研究基于转录组分析结果, 剖析了解除种子休眠过程中显著性差异表达unigenes的变化, 进而挖掘获得了ABA合成途径、代谢途径中的关键基因AhNCED2和AhCYP707A1的基因片段序列, 通过功能验证分析, 以期为培育具有适度休眠的花生品种提供理论指导。
选用山东省花生研究所育成的小花生品种花育52和花育28。花育52具有强休眠特性, 花育28具有弱休眠特性。以花育52吸胀24 h (28℃)的休眠种子为对照(CK), 用100 mg L-1乙烯利处理对照2.5h后取生长不同时间的种子用于转录组测序(表1)。
花育52休眠种子(HY52-D)、花育52室温破除休眠的种子(HY52-ND)、花育28无休眠种子(HY28), 取样时间为吸胀0、6、12、18和24 h的种子, 以及对照CK、AE1、AE2和AE3 (表1)用于验证分析基因的表达差异。
| 表1 供试材料 Table 1 The tested materials |
| 附表1 种子休眠解除过程中与ABA、GA、ETH、auxin相关的显著性差异表达unigenes Supplementary table 1 Significant differential expression unigenes associated with GA, ABA, ETH, and auxin during the process from dormancy to germination |
采用Illumina/Solexa标准操作步骤(Directional mRNA-Seq Sample Preparation Part # 15018460 Rev. A, October 2010)制备转录组测序文库。其后用Agilent2100对文库建库片段大小进行质控, 采用
Illumina 2000平台对文库进行测序。
对所产生的原始reads进行质量评估和可信度分析, 去除低质量片段(Q< 20)。使用软件Trinity (trinityrnaseq_r2012-10-05)将花生4个样本的有效reads合并进行de novo拼接, 获得121412个Unigene。将获得的Unigenes进行注释, 包括NR、Swiss-Prot等基本数据库注释、KOG分类、GO和KEGG注释。
使用RPKM (Reads per kb per million reads)计算基因表达量; 根据unigenes表达量采用fold change分析、fisher检验、chisq检验等进行差异表达分析。样本AE1、AE2、AE3均与CK相比较, 取值P≤ 0.05且|fold change|≥ 2。
按照RNA试剂盒(TransGen)说明书提取花生种子总RNA, 用Prime RT Reagent Kit (TaKaRa, 大连)将提取的总RNA反转录成cDNA。
采用LightCycle 2.0 (rRoche Diagnostics公司)荧光定量PCR仪。PCR程序为95℃ 30 s; 95℃ 5 s, 60℃ 20 s, 72℃ 10 s , 45个循环; 然后绘制溶解曲线。采用SYBR Premix Ex Taq试剂盒(TaKaRa, 大连), 按照说明进行实时定量PCR。每个样品重复3次, 取平均值, 采用2-Δ Δ Ct的方法。根据目的基因片段序列, 利用Beacon Designer 7.91软件设计荧光定量PCR引物, 内参基因为Actin11(表2)。
利用DNA MAN 6.0对目的基因片段的氨基酸序列、开放阅读框搜索进行分析; 利用在线Blast (http://www.ncbi.nlm.nih.gov/Blast/)进行序列比对、同源序列搜索。
| 表2 引物序列 Table 2 Primer sequence |
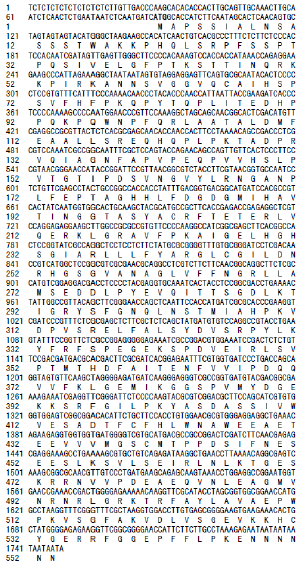
| 附表2 花生AhNCED2基因片段和AhCYP707A1基因片段的核苷酸序列和推测氨基酸序列 Supplementary table 2 Nucleotide and pupative AA sequences of the AhNCED2and AhCYP707A1gene fragment |
基于转录组分析结果, AE1、AE2、AE3与吸胀休眠种子(CK)相比较, 3组对比数据中至少有一组数据满足|fold change|≥ 2且P≤ 0.05要求。通过比较获得了40个与ABA、15个与GA、60个与ETH、56个与auxin相关unigenes的表达存在显著差异(附表1)。
NCED是ABA合成途径中的关键酶, CYP707A是ABA代谢途径中的关键酶。在外源乙烯利作用下花生种子休眠解除过程中, 检测到2个NCED的unigenes, 其表达下调。4个CYP707A unigenes的表达有一定差异, AE1表现显著上调表达。外源乙烯利作用下花生种子休眠解除过程中, 检测到8个ABA受体(PYL) unigenes, 其中4个在AE1表现显著上调, 6个在AE2表现显著上调; 检测到6个ABA响应因子(ABF) unigenes均表现下调, 特别在种子露白阶段均显著下调; 检测到20个可能的PP2C unigenes, 其表达表现较为复杂(图1和附表1)。
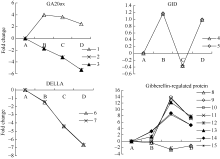
GA20ox是GA合成途径的关键基因, 花生种子休眠解除过程中, 检测到1个GA20ox的unigene表达显著上调, 2个GA20ox1-like的unigenes表达显著下调。GA信号转导途径中, GID1是GA受体, GID1 unigenes的表达受到外源乙烯利诱导; DELLA蛋白是一类核蛋白, 属于转录调控因子GRAS家族[28], 是GA信号传导中非常重要的抑制因子, 外源乙烯利作用下花生种子休眠解除过程中, DELLA蛋白unigenes均表现下调, 在种子露白阶段(AE2)均显著下调。检测到8个GA调控蛋白unigenes, AE1均未达到显著差异表达, AE2 (种子露白)时6个表现显著上调、2个表现显著下调(图2和附表1)。
植物体内乙烯通过Yang Cycle由蛋氨酸转换产生[29, 30], ACS和ACO是乙烯生物合成途径中的关键限速酶。外源乙烯利作用下吸胀休眠种子萌发过程中, 检测到60个与ETH相关的unigenes (图3, 附表1)。外源乙烯利作用下, ACS unigenes迅速响应, AE1处理上调表达10倍以上, 露白时上调表达10倍以上; 检测到12个差异表达的ACO unigenes, 它们均是在种子露白时表达达到峰值, 且都是显著上调表达, 差异倍数变幅5.099~14.661。另外检测到参与乙烯信号转导途径中的unigenes有EIN2 unigenes 2个、6个ETH受体(ETR和ERS) unigenes、6个EBF1-2 unigenes、33个ETH响应因子(EREBP-like) unigenes。外源乙烯利作用下, EIN2 unigenes表达显著下调, 随即表达量有所上升但均未达到显著水平; ETH受体unigenes表现上调表达; EBF是负责识别及结合EIN3等转录因子的F-box蛋白, 我们检测到6个EBF1-2 unigenes, 它们均表现显著上调; ETH响应因子unigenes表达模式有差异, 8个在AE1处理达表达峰值, 10个在AE2处理达表达峰值。
 | 图1 ABA相关unigenes在种子休眠解除过程中的表达变化数字对应unigenes见附表1。 A: log2(CK_RPKM/CK_RPKM); B: log2(AE1_RPKM/CK_RPKM); C: log2(AE2_RPKM/CK_RPKM); D: log2(AE3_RPKM/CK_RPKM). Number corresponding to unigenes see Supplementary table 1.Fig. 1 Expression trend of unigenes related to ABA during the process of seed dormancy release |
 | 图2 GA相关unigenes在种子休眠解除过程中的表达变化数字对应unigenes见附表1。Fig. 2 Expression trend of unigenes related to GA during the process of seed dormancy releaseA: log2(CK_RPKM/CK_RPKM); B: log2(AE1_RPKM/CK_RPKM); C: log2(AE2_RPKM/CK_RPKM); D: log2(AE3_RPKM/CK_RPKM). Number corresponding to unigenes see Supplementary table 1. |
 | 图3 ETH相关unigenes在种子休眠解除过程中的表达变化数字对应unigenes见附表1。Fig. 3 Expression trend of unigenes related to ETH during the process of seed dormancy releaseA: log2(CK_RPKM/CK_RPKM); B: log2(AE1_RPKM/CK_RPKM); C: log2(AE2_RPKM/CK_RPKM); D: log2(AE3_RPKM/CK_RPKM). Number corresponding to unigenes see Supplementary table 1. |
生长素(auxin)作为一类重要的激素, 参与细胞的分裂、生长、成熟和分化等过程。外源乙烯利作用下吸胀休眠种子萌发过程中, 检测到与auxin相关的unigenes 56个(图4和附表1), 包括生长素诱导蛋白、生长素响应蛋白、SAUR家族蛋白、AUX1 LAX家族、生长素抑制蛋白、生长素响应因子等。其中生长素抑制蛋白unigenes在种子休眠解除过程中显著下调表达; 生长素诱导蛋白和生长素响应GH3 unigenes在种子休眠解除过程中的表达有分化; 其他检测到与auxin相关基因的表达均在AE2种子露白时达到峰值, 且显著上调表达。
通过转录组测序, 得到了NCED基因cDNA的部分序列(附表2), 序列长度为1748 bp。用DNAMAN软件分析该cDNA序列, 发现其5° 端有88 bp的非编码区(5° -UTR), 编码553个氨基酸, 没有终止密码子; 将该基因片段推导的氨基酸序列与AhNCED1 (CAE00459.2)比对分析, 两者同源相似性仅为53.90%, 暂定名为AhNCED2; 进一步在NCBI上进行Blastp比对, 表明该基因推导的氨基酸序列与其他植物的NCED氨基酸序列相似性较高, 包含RPE65保守结构域(图5), 其中与鹰嘴豆(Cicer arietinum, XP_004504912.1)的相似性最高, 达到79%, 与豌豆(Pisum sativum, BAC10551.1)、大豆(Glycine max, NP_001241616.1)、金钱橘(Citrus clementina, ABC26010.1)、温州蜜柑(Citrus unshiu, BAE92960.1)、烟草(Nicotiana tabacum, AFP57678.1)、马铃薯(Solanum tuberosum, AAT75152.1)、拟南芥(Arabidopsis thaliana, NP_188062.1)等的相似性分别为 73%、77%、71%、72%、71%、70%和73%。
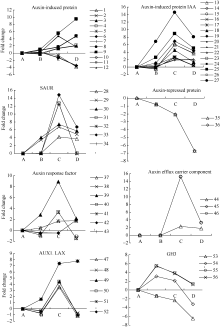 | 图4 auxin相关unigenes在种子休眠解除过程中的表达变化数字对应unigenes见附表1。Fig. 4 Expression trend of unigenes related to auxin during the process of seed dormancy releaseA: log2(CK_RPKM/CK_RPKM); B: log2(AE1_RPKM/CK_RPKM); C: log2(AE2_RPKM/CK_RPKM); D: log2(AE3_RPKM/CK_RPKM). Number corresponding to unigenes see Supplementary table 1. |
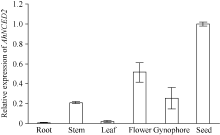
利用荧光定量技术检测AhNCED2基因在解除吸胀花生种子休眠及萌发过程中的表达差异(图6)。花育52具有强休眠性, AhNCED2在吸胀休眠态种子(CK)中表达量最高, 随着种子休眠解除、萌发开始, AhNCED2的表达量呈下降趋势。AhNCED2在不同品种吸胀萌发过程中的表达差异很大, AhNCED2在HY52-D (休眠种子)吸胀过程中表达上调, 吸胀18 h时表达量达到最高, 上调10倍以上, 随后下降; HY52- ND (花育52破休眠种子)和HY28 (花育28无休眠种子)种子吸胀过程中AhNCED2的表达下调, 干种子中的表达量最高(图7)。组织特异性表达表明AhNCED2基因种子中的相对表达量最高, 其次是花、果针、茎、叶, 根中AhNCED2基因的表达量最低(图8)。
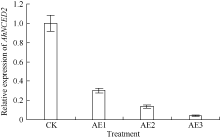 | 图s6 乙烯利处理花育52休眠态过程中AhNCED2基因的相对表达水平Fig. 6 Relative expression of AhNCED2 at breaking Huayu 52 seed dormancy by ethephon |
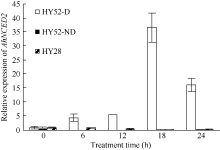 | 图7 不同品种吸胀萌发过程中AhNCED2基因的相对表达水平Fig. 7 Relative expression of AhNCED2gene at different dormant stages of different varieties |
通过转录组测序, 得到了CYP707A1基因片段的cDNA 序列(附表2), 序列长度为1672 bp。用DNAMAN软件对该cDNA序列进行分析, 发现其5° 端有349 bp的非编码区(5° -UTR), 编码442个氨基酸。将该序列在NCBI网站上进行在线Blastp分析表明, 该基因推导的氨基酸序列与花生ABA 8’ 羟化酶(CDJ80018.1)的氨基酸序列相似性为98%, 证实该序列为花生ABA 8’ 羟化酶基因片段, 名称为AhCYP707A1。在NCBI上进行BlastP比对, 表明该基因片段中包含P450保守结构域(图9)。
与其他植物的ABA 8’ 羟化酶基因氨基酸序列相比相似性很高, 与大豆(Glycine max, NP_001237490.1)、蒺藜苜蓿(Medicago truncatula, XP_003629019.1)、菜豆(Phaseolus vulgaris, ABC86558.1)、拟南芥(Arabidopsis thaliana, NP_974574.1) ABA 8’ 羟化酶的氨基酸序列相似性分别为81%、82%、82%和73%。
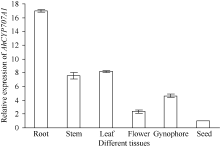
利用荧光定量PCR技术检测AhCYP707A1基因在外源乙烯利解除吸胀花生种子休眠及萌发过程中的表达差异(图10)。AhCYP707A1在吸胀休眠态种子(CK)中表达量最低, 与休眠态相比, 解除休眠种子萌发过程中AhCYP707A1基因的表达上调10倍以上, 其中AE1处理表达量最高。HY52-D种子吸胀0、6、12、18和24 h样品中AhCYP707A1的表达量略有上升, 差异表达倍数分别为1.64、1.95、1.61和1.98倍; HY28种子吸胀萌发过程中, AhCYP707A1的表达量急剧上升, 与干种子相比, 6、12、18和24 h样品中AhCYP707A1的表达量差异表达倍数在10倍以上; HY52-ND种子吸胀萌发过程中, AhCYP707A1的表达量呈上升趋势, 与干种子相比, 6、12、18和24 h样品中AhCYP707A1的差异表达倍数分别是1.99、4.42、5.50和8.82倍(图11)。AhCYP707A1基因在花生不同组织的表达差异性分析表明, AhCYP707A1基因在根中的相对表达量最高, 其次是在叶、茎、果针和花, 种子中的相对表达量最小(图12)。
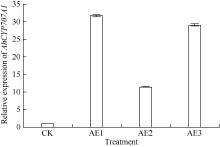 | 图10 乙烯利处理花育52休眠态过程中AhCYP707A1基因的相对表达水平Fig. 10 Relative expression of AhCYP707A1 at breaking Huayu 52 seed dormancy by ethephon |
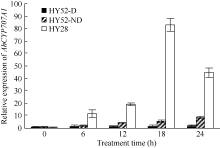 | 图11 不同品种吸胀萌发过程中AhCYP707A1基因的相对表达水平Fig. 11 Relative expression of AhCYP707A1 gene at different dormant stages of different varieties |
大量研究表明ABA是种子休眠诱导的正调节因子和萌发的负调节因子。过量表达ABA生物合成基因能增加种子中的ABA含量, 从而促进种子休眠或者延迟萌发[31, 32]。NCED是ABA合成途径中的关键限速酶。拟南芥中有5个NCED基因, NCED6和NCED9在发育种子中高效表达, NCED6在胚乳中特异性表达, NCED9在胚和种皮中表达。大麦中, 2个HvNCED基因调控种子成熟期ABA积累[33]。CYP707A是调控ABA代谢的关键限速酶。拟南芥ABA代谢缺陷型突变体中编码ABA 8’ 羟化酶的基因(CYP707A1、CYP707A2)失活会导致种子休眠性增强[8, 9]。休眠种子和无休眠种子吸胀时ABA含量均下降; 但休眠种子(拟南芥和大麦)中维持较高水平ABA含量、CYP707A转录水平表达量较低[34]。ABA缺乏的突变体易形成非休眠种子或种子易在母体植株上提早萌发[14, 35, 36]。
种子在吸胀过程中, 无论是休眠种子还是非休眠态种子, 其内部生理生化和代谢水平均在发生巨大变化。休眠花生种子吸胀过程中, ABA合成关键基因AhNCED2表达量上调以维持种子休眠特性, 吸胀18 h时达到峰值, 随即开始下降。当以外源乙烯利处理吸胀休眠态的种子时, AhNCED2表达量随休眠解除和萌发进程下降; 无休眠种子吸胀过程中AhNCED2在干种子中表达量最高。ABA代谢途径关键基因AhCYP707A1, 在休眠种子和非休眠态种子吸胀过程中均上调, 但非休眠种子表达量高, 上调倍数高; 外源乙烯处理吸胀休眠种子时, AhCYP707A1迅速上调表达。外源乙烯利作用下花生种子休眠解除过程中, 检测到6个ABA信号转导途径相关的ABA响应因子unigenes均下调表达, 特别在种子露白阶段均显著下调。这些结果进一步推测外源乙烯利对吸胀休眠态种子的ABA有影响, 一是ABA合成关键基因AhNCED2下调表达, 切断了ABA合成途径; 二是ABA代谢途径中AhCYP707A1上调表达, 降解ABA以使种子中ABA含量减少; 三是对ABA信号途径中ABA响应因子有诱导作用。
种子萌发过程中GA与乙烯间存在互作, 山毛榉种子受到乙烯利处理时GA20ox1表达上调[37]。GA20ox是GA合成途径的关键基因, 花生种子休眠解除过程中, 检测到1个GA20ox的unigene和2个GA20ox1-like 的unigenes, 外源乙烯利处理下GA20ox的表达表现出多样性, 其中1个表现显著上调, 另外2个GA20ox1-like的unigenes表现显著下调。DELLA蛋白是一类核蛋白, 属于转录调控因子GRAS家族[28], 是GA信号传导中非常重要的抑制因子, 外源乙烯利作用下花生种子休眠解除过程中, DELLA蛋白unigenes下调, 在种子露白阶段显著下调。GA调控蛋白unigenes, AE1处理无显著差异表达, AE2处理(种子露白) 6个显著上调、6个显著下调。
植物体内乙烯由Yang Cycle途径产生[29, 30], ACS和ACO是乙烯生物合成途径中的关键限速酶。种子萌发过程中, 乙烯生物合成和信号途径是胚乳弱化和破裂所必需的, ACO是控制乙烯合成的关键因子[12, 38, 39, 40, 41]。在豌豆种子萌发过程中, 乙烯通过Ps-ACO1转录物的正反馈调节促进乙烯的生物合成, 而Ps-ACS1 mRNA的水平和ACC的总含量不被乙烯处理影响。乙烯对豌豆种子萌发的促进作用与胚轴胚根处的β -1, 3-葡聚糖酶有关[40, 42]。本研究表明外源乙烯利处理对乙烯合成途径ACS和ACO基因均有影响, ACS unigenes表达响应早于ACO unigenes, ACO unigenes在种子露白时达表达峰值, 推测ACO与花生种子萌发密切相关。外源乙烯利对乙烯信号转导途径中的unigenes有影响, ETH受体上调表达、负责识别及结合EIN3等转录因子的 F-box蛋白显著上调, 而ETH响应因子unigenes的表达模式复杂, 8个迅速响应、10个在种子露白时达表达峰值。
生长素(auxin)作为一类重要的激素, 参与细胞的分裂、生长、成熟和分化等过程[43, 44, 45]。生长素也是诱导和维持种子休眠所必需的, 主要证据来自生长素信号缺失突变体的种子休眠性不同程度减弱、生长素合成缺陷突变体的休眠性减弱、生长素合成过量突变体的种子休眠性得到显著提高[46]。外源乙烯利作用下种子休眠解除过程中, 生长素抑制蛋白unigenes显著下调表达、生长素诱导蛋白和生长素响应GH3 unigenes、表达有分化、其他与auxin相关的unigenes在种子露白时达到峰值, 且是显著上调表达。
种子萌发过程中, ETH和ABA存在拮抗作用[39, 47, 48]。GA-乙烯协同作用促进种子休眠解除、后熟和发芽[49]。Lepidium sativum[41]和糖甜菜[38]中, 外源乙烯或ACC对ABA含量及ABA合成基因表达也没有影响。然而, 与野生型相比, 拟南芥乙烯不敏感突变体etr1和ein2含有高含量ABA, 萌发缓慢[49, 50, 51]。乙烯不仅作用于ABA代谢降低ABA含量, 也负向调控ABA信号途径[52, 53]。本研究中外源乙烯利处理下, NECD、CYP707A、ABF、DELLA、ACS、EBF1-2、EREBP-like (8个)、ERS、ETR受到明显诱导, 而PYL、ACO、GA调控蛋白、EREBP-like (10个)以及多数auxin相关unigenes的表达峰值与花生种子露白时一致。
Liu等[54]克隆了花生AhCYP707A1、AhCYP707A2、AhNCED1基因, AhCYP707A1和AhCYP707A2基因均在根中高表达。LiCl胁迫对AhCYP707A1、AhCYP707A2、AhNCED1基因在根、茎、叶中的表达均没有影响; PEG-6000和NaCl胁迫诱导AhCYP707A2基因在根、茎、叶中的表达明显上调, 诱导AhNCED1基因在茎、叶中的表达上调, 诱导AhCYP707A1基因仅在根中表达上调。本研究获得的NCED基因片段与AhNCED1 (CAE00459.2)的比对发现两者氨基酸序列的同源相似性为53.90%, 推测两者不是同一个基因, 暂定名为AhNCED2, 组织特异性表明AhNCED2基因种子中的相对表达量最高。另外获得了CYP707A1基因片段与花生AhCYP707A1 (CDJ80018.1)的氨基酸序列相似性为98%, 推测两者可能是同一个基因, 组织特异性表明该基因根中的相对表达量最高, 与Liu等的研究结果一致。荧光定量PCR验证表明AhNCED2和AhCYP707A1受到外源乙烯利的诱导, 与花生种子休眠维持及解除密切相关。
外源乙烯利诱导花生种子休眠解除过程中, 与GA、ABA、ETH及auxin相关unigenes表现显著差异表达, 表明外源乙烯利通过GA、ABA、ETH、auxin相关基因的诱导作用来完成其对花生种子休眠的解除。AhNCED2和AhCYP707A1受外源乙烯利的诱导, 与花生种子休眠的维持及解除密切相关, 为培育具有适度休眠性的花生新种质提供了候选基因。
The authors have declared that no competing interests exist.
作者已声明无竞争性利益关系。The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|