第一作者联系方式: E-mail: lcn560@163.com
甘蔗是经济和环境上日益重要的C4作物, 干旱在全球范围内严重限制甘蔗产量。了解甘蔗对水分胁迫反应的分子机制将有助于甘蔗抗旱性的分子遗传改良。利用基因芯片技术分析水分胁迫下甘蔗叶片的15 593个基因的表达谱, 结果表明, 中、重度胁迫下的差异表达基因数量分别为300个和853个, 中度胁迫中差异基因以上调表达为主, 重度胁迫中下调表达占多数。功能注释分析显示, 差异表达基因分子功能主要为结合、载体和催化活性, 主要参与代谢、细胞和生物调控等生物过程。此外, 功能未明确的假定蛋白和无匹配信息的基因序列仍占据注释结果的相当一部分, 表明还有大量的基因尚待发掘。在水分胁迫下, 甘蔗内源ABA和IAA含量显著上升而GA含量显著受到抑制。以参与生物进程分类, 对植物激素相关基因进行筛选并分析, 发现激素响应表达基因代谢途径具有多样性, 显示了激素代谢网络的交叉性与复杂性。挑选9个差异表达程度不同的基因进行实时荧光定量PCR检测, 表明芯片数据具有良好的重复性。
Sugarcane is an increasingly economically and environmentally important C4 crop. Water stress limits enormously sugarcane productivity worldwide, and understanding the molecular mechanisms for sugarcane stress responses will be useful for sugarcane improvement by genetic manipulation. To investigate the transcriptome changes in response to water stress, we used microarrays to profile expressions of 15 593 genes in sugarcane exposed to drought. The results indicated that 300 and 853 differentially expressed genes were detected under moderate and severe water stresses, respectively. The expression of differentially expressed genes treated with moderate water stress was mainly up-regulated, however that treated with severe water stress was mainly down-regulated. To further characterize these genes, we used Gene Ontology (GO) for their annotation, the results showed that differentially expressed genes possessed the functions of binding, transporter, molecular transducer and catalytic activities and were involved in metabolic, biological regulation and cellular processes. Besides, hypothetical protein and no match annotated results were found to fill a large part of those genes, indicating that effective approach should be adopted to discover novel genes in sugarcane genomics. Water stress resulted in an increase in ABA and IAA contents but a depression in GA content. Classified by biological process, 46 plant hormone related genes were selected, further annotation analysis showed that the metabolic pathways of some plant hormone responsive genes were diverse or had crosstalk with each other, indicating the intersectionality and complexity of plant hormone signaling pathway. Additionally, the relative expressions of nine selected genes were validated by quantitative Real-time PCR (qRT-PCR), further confirming the reliability of microarray results.
甘蔗为重要的糖料和能源作物, 在维持社会可持续发展中扮演重要角色。干旱致使甘蔗减产和品质降低[1]。作物能感受刺激和传递信号, 启动各种生理生化反应以响应和适应水分胁迫[2]。植物内源激素作为化学信号参与调节众多植物生长发育代谢进程, 并在适应环境胁迫中发挥重要作用[3, 4]。在水分胁迫下, 脱落酸(ABA)可诱导气孔关闭, 减少蒸腾量及水分散失[3]; 细胞分裂素参与植物对水分胁迫的响应并通过与ABA协作而实现。在渗透胁迫下, 随着ABA含量增加, 与ABA具有拮抗作用的细胞分裂素含量下降, 气孔得以顺利关闭, 水分蒸腾量随之减少[5]; 赤霉素对逆境胁迫的反应与生长素类似, 就是含量降低, 植物正常生长发育受阻[6]; 水分胁迫下植物体内乙烯浓度急剧增加[7], 呼吸作用增强、衰老加速、成熟加快[8], ROS导致的膜脂过氧化作用与乙烯浓度密切相关[9], 逆境下乙烯合成量与植物耐胁迫能力呈负相关[7]。单一植物激素生理功能的实现离不开其他类激素的协同作用, 进而通过影响激素合成和信号转导关键基因的表达及分子转导, 调节各激素的含量水平[10, 11], 从而实现激素的相互调节, 这已经成为植物生长发育中一种重要的调控手段[12, 13]。
基因差异表达谱为植物在特定环境及生理状态下的一个基因表达汇总。应用基因芯片筛选逆境胁迫下差异表达基因, 分析基因的功能, 不仅能使我们更好地理解作物响应及耐受胁迫的分子机制, 也为利用这些基因对作物进行定向遗传改良以提高作物的抗胁迫能力奠定一定的基础[14, 15]。通过该技术, 前人已从各处理条件下的多种作物的转录水平上挖掘出许多的差异表达基因并应用于作物分子改良[16, 17, 18, 19, 20]。本研究目的在于利用基因芯片技术分析水分胁迫下甘蔗叶片中基因表达谱的差异, 并对激素相关基因作进一步分析, 探讨甘蔗水分胁迫响应的激素调节分子基础。
试验材料为耐旱品种桂糖21号(GT21)。蔗种以单芽方式种植于泥沙混合培养基质上, 50 d后, 选取长势一致的甘蔗苗移栽至桶中, 每桶2~3株。桶规格约30 cm× 35 cm (直径× 高), 每桶装混合土18 kg (土∶ 有机肥∶ 沙=70∶ 20∶ 10, w/w), 桶底钻孔以增强透气性。把桶移至智能温室大棚, 日常管理, 使其正常生长5个月处于伸长盛期时, 把材料分为2组。第一组正常淋水, 为对照, 第二组停止淋水, 分别于断水5 d和7 d后取样, 清晨采集+1叶样品, 速冻于液氮中, 带回实验室-80℃冰箱保存待分析。处理期间, 对照及处理土壤绝对含水量分别为20%± 2%和9%± 2%。
称取新鲜甘蔗叶片0.5 g, 用80%预冷甲醇置弱光下冰浴研磨至匀浆, 4℃避光放置24 h, 中间振荡混匀4~5次, 10 000× g冷冻离心15 min, 取上清液过C-18柱, 真空干燥后, 以样品稀释液(含0.1% Tween-20和0.1%明胶的磷酸盐缓冲液, pH 7.5)溶解即得样品激素提取液[21]。采用酶联免疫吸附法(ELISA)按说明书测定ABA、IAA和GA的含量, 酶联免疫试剂盒购于中国农业大学, 使用ANTHOS- 2010酶标仪测定吸光值, 每样品重复测定3次, 取平均值。
选用Agilent 4× 44K规格芯片。甘蔗基因数据来自NCBI的Unigene库, 共包含有代表不同基因序列的15 593条探针。依照Agilent基因表达谱芯片(单标)操作指南, 使用TRIzol法提取叶片的总RNA并纯化, 以T7 Promotor primer为引物配制cDNA合成体系, 应用一步法合成cDNA的第1和第2链。以上述cDNA为模板并添加aa-UTP, 在T7 RNA聚合酶作用下合成cRNA, 经纯化及浓度测定后的cRNA加到荧光染料(Cy3)中进行标记, 纯化标记好的cRNA, 加到片段化溶液中进行样品片段化。
取100 µ L片段化样品上芯片, 65℃滚动杂交17 h (10 转 min-1), 杂交完毕, 取出芯片于洗涤液中清洗干净待扫描。采用Agilent扫描仪来获取图像, 分辨率为5 μ m, 扫描仪自动以100%和10% PMT各扫描一次, 以GeneSpring软件读取原始数据, Agilent软件可自动合并2次结果。
采用Aligent Scanner获取图像, 通过Imagene转化为数值后应用Genespring软件将数值标准化, 取得Ratio值。之后对芯片的杂交数据进行比较, 差异表达基因筛选标准为表达倍数Fold change≥ 2或≤ 0.5 (表达上调或下调), FDR (False discover rate)< 0.05, 即q < 0.05, 且方差分析的P≤ 0.01, 以上3个条件必须同时满足。使用AmiGO的Blast Query在线软件对筛选出的差异基因进行Gene Ontology分析, 取舍阈值为比对E-value≤ 1E-20。通过BlastX将参与植物激素生物过程的基因序列比对到蛋白数据库(E-value≤ 1E-20), 得到该基因功能注释信息。
随机挑选9个筛选出来的差异表达基因, 设计特异引物, 提取同期样品RNA, 逆转录为cDNA, 进行实时荧光定量PCR分析(表1), 选用GAPDH基因为内参[22]。反应体系含10 μ L 2× All-in-One qPCR Mix (Gene Copoeia)、2 μ L cDNA、4 μ mol L-1的正反向引物各1 μ L、6 μ L双蒸水。程序为95℃预变性10 min; 95℃变性10 s, 60℃退火20 s, 72℃延伸20 s, 40个循环。反应完成后进行熔解曲线检验, 采用2-Δ Δ Ct法进行相对表达量计算[23]。
| 表1 实时定量PCR选用基因及引物 Table 1 Genes and primers list for qRT-PCR |
水分胁迫处理提高了甘蔗叶片(图1) ABA和IAA含量, 在处理第5、第7天, 两者分别比对照增加32.3%、9.1%和71.8%、19.7%, 增量显著; 相反, GA含量显著受到水分胁迫的抑制, 处理第5、第7天, 其含量分别比对照低15.2%和26.5%。
Agilent单通道表达谱芯片用重复探针点(10次重复)信号的变异系数(CV值)来计算芯片的稳定性, 其质控标准是CV值小于15%。本次12张基因芯片(每处理3个重复)的CV值为5.23%~8.15%, 芯片检出率在93.22%~ 94.26%之间, 表明结果合格可用。经筛选, 水分胁迫处理第5天的差异表达基因数量为300个, 上、下调表达基因数量分别为175个和125个; 从第7天的样品中筛选到差异表达基因853个, 上、下调表达基因数量分别为418个和435个; 第5、第7天共有的差异表达基因数目为183个(图2-A)。
分别对筛选出来的差异表达基因进行功能注释和GO分析, 其中第5、第7天183个共有差异表达基因中, 获得功能注释的基因数为103个, 编码假定蛋白的基因数为58个, 22个基因未找到匹配信息, 3种注释情况占比分别为56.3%、31.7%、12.0%, 除此以外, 第5、第7天特有差异表达基因3种注释情况占比分别为55.6%、31.6%、12.8%和63.6%、20.7%、15.7% (图2-B)。第5、第7天183个共有差异表达基因中, 获得有效GO注释基因118个, 占比为64.5%, 其余两组基因的注释数量分别为67个和420个, 占比为57.3%和62.7% (图2-C)。118个第5、第7天共有差异表达基因共获得369条GO注释信息, 生物进程、细胞组分和分子功能分别占61.5%、10.6%和27.9%, 处理第5、7天特有的67和420个基因分别获得263和930条GO注释信息, 两组基因的生物进程、细胞组分和分子功能分别占70.7%、9.1%、20.2%和64.7%、10.5%、24.7% (图2-D)。
以生物进程分类, 筛选出的差异表达基因参与的生物进程主要有细胞过程(cellular process), 代谢过程(metabolic process)、应激反应(response to stimulus)、生物调节(biological regulation)、生物过程调节(regulation of biological process)、发育过程(developmental process)、定位(localization)、多器官进程(multi-organism process)等; 以细胞组分分类, 差异基因被归于细胞组件(cell part)、细胞器(organelle)、大分子复合体(macromolecular complex)和膜关闭内腔(membrane-enclosed lumen); 以分子功能分类, 差异基因被归于催化活性(catalytic activity)、载体活性(transporter activity)、结合活性(binding)、分子转导活性(molecular transducer activity)(图3)。
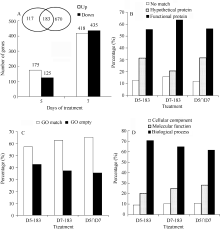 | 图2 水分胁迫下甘蔗品种GT21叶片中差异表达基因筛选和功能注释Fig. 2 Screening and annotation of differential expression genes in sugarcane cultivar GT21 under water stress |
以参与生物进程分类, 对植物激素相关基因进行筛选。第5、第7天共有差异表达基因9个(表2), 其中6个上调、3个下调表达, 第5、第7天特有差异表达基因分别为6 (表3)和31个(表4), 上调表达数量分别为4个和14个, 下调表达数量为2个和17个, 所编码的基因产物包括蛋白磷酸酶(CA093454, CA078060, CA282798)、蛋白激酶(CA160805, CA285332, CA280103, CA135993)、受体蛋白(CA252520, CA076654, CA138168), 转运蛋白(CA151192, CA279772, CA080185, CA106306, CA186908)等, 这些基因参与了包括赤霉素、生长素、细胞分裂素、脱落酸、乙烯的生物合成、代谢、转运、响应或信号转导通路等生物进程。
| 表2 水分胁迫第5、第7天共有的激素相关差异表达基因功能注释和GO分析 Table 2 Annotation and GO terms of genes related to phytohormone pathways in both 5th and 7th day under water stress |
| 表3 水分胁迫5 d特有的激素相关差异表达基因功能注释和GO分析 Table 3 Annotation and GO terms of genes related to phytohormone pathway in 5th day under water stress |
| 表4 水分胁迫7 d特有的激素相关差异表达基因功能注释和GO分析 Table 4 Annotation and GO terms of genes related to phytohormone pathway in the 7th day under water stress |
作物的抗旱性是由多基因控制的复杂数量性状, 涉及信号转导、编码保护、防御和胁迫耐受蛋白、能量代谢、膜运输和转录翻译等方面[24, 25]。干旱条件的复杂性和作物基因型的多样性决定了作物对干旱的适应性存在多条途径, 不同强度或类型的水分胁迫很可能导致不同程度或方式的适应性[26, 27]。本研究采用基因芯片技术构建甘蔗品种GT21在中度和重度水分胁迫下叶片基因表达谱数据库。结果显示, 在重度水分胁迫下差异表达基因数量远高于中度胁迫, 中度胁迫差异基因以上调表达为主, 重度胁迫差异基因下调表达占多数, 这也许与上述胁迫适应调节机制密切相关, 但仍需进一步研究揭示。
作物在形态结构和生理生化水平上的抗旱性, 都是内在相关基因表达调控的结果[24, 25]。水分胁迫下, 作物细胞迅速感知外界信号, 通过信号转导、基因转录、转录后3个水平调节, 激活胁迫应答基因的表达, 大量特异蛋白得以生成, 协同作用调节植物体内的生理生化和代谢水平, 提高作物抗旱性[28, 29]。本研究对筛选出来的差异基因进行功能注释和GO分类, 结果显示这些基因参与的代谢进程有脂类代谢、信号转导作用、蛋白质代谢、ABA代谢、光合作用、碳水化合物代谢、细胞代谢、呼吸作用、能量代谢等, 或者是编码蛋白激酶、渗透调节物质、转录因子、碳水化合物和次生代谢相关酶类等, 表明甘蔗对水分胁迫适应代谢途径的多样性和复杂性。由于甘蔗的遗传背景和基因组的复杂性, 迄今为止还尚未见有关其全基因组测序的报道, 功能未明确的假定蛋白和无匹配信息的基因序列仍占据注释结果的相当一部分, 表明进一步揭示甘蔗响应水分胁迫的详细机理仍需大量的研究工作。
水分胁迫可以引起作物一系列的生理生化反应, 其中植物内源激素含量的变化及相互间的平衡调节是植物响应水分胁迫的重要生理活动[10, 11, 12, 13, 30]。大量研究表明, 脱落酸(ABA)、吲哚乙酸(IAA)、赤霉素(GA)等内源激素在植株受水分胁迫期间起着信号传导并主动适应的作用, 直接影响作物对逆境的适应性, 反映植株对干旱胁迫的响应程度[31]。水分胁迫会促使植物的内源ABA含量增加, 作为信号分子ABA可激活多种离子通道以及相关的生理生化反应酶类, 促进气孔关闭, 降低蒸腾速率, 减少水分散失, 进而减少干旱对植物的伤害[32]。胁迫初期植物内源IAA合成会减少以减缓生长, 随着胁迫程度的增强, 由于侧根和不定根的生长需要, 内源IAA合成量可能增多, 但内源IAA含量变化因作物种类而定, 具体机制较为复杂[33, 34]。IAA、GA这类与作物生长发育相关植物激素含量降低, 使植物生长速率减慢, 从形态、生理等各方面发生相应变化, 以提高自身的抗旱力, 减轻逆境造成的伤害。本研究表明, 水分胁迫下, 随着ABA含量显著上升, 甘蔗叶片中的IAA含量也随着上升, 而GA含量显著受到抑制, 显示了ABA、IAA和GA在甘蔗响应逆境胁迫时的重要联系。
植物对逆境胁迫的响应主要有ABA依赖和非ABA依赖两条代谢途径[35]。本研究对筛选出来的激素相关的46个基因作进一步分析发现, 与ABA响应相关的有26个基因, 其中与ABA信号转导有关的SnRK2基因(CA285332, CA280103), PP2Cs基因(CA093454, CA078060)。在正常条件下, PP2Cs可与SnRK2s结合, 使SnRK2s发生去磷酸化作用而失活; 在水分胁迫下, 植物内源ABA的含量急剧增加, ABA与受体蛋白结合后, 再与PP2C相互作用, PP2C构象改变, 其酶活性受到抑制, 受PP2C负调控的SnRK2酶活性得以释放[36, 37], SnRK2的作用使下游成员, 包括AREB等转录因子磷酸化[38, 39], 最终激活响应ABA信号靶基因的表达, 实现植物对水分胁迫响应的调节[40, 41]。此外, ABA响应表达基因代谢途径具有多样性[42, 43]。本研究注释结果显示, 上述PP2Cs基因不仅与ABA信号转导有关, 还参与生长素和乙烯的响应进程; G-box结合因子(BU103681)、syntaxin 121 (CA123082)、蛋白激酶(CA160805)、ABC转运蛋白(CA151192、CA080185)等基因也参与众多激素响应代谢进程, 表明激素代谢网络的交叉性与复杂性。
The authors have declared that no competing interests exist.
作者已声明无竞争性利益关系。The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|





