叶片衰老是作物叶片发育的最后阶段, 功能叶早衰将影响作物产量和品质, 因此, 研究叶片早衰的分子与生理机制对于培育耐早衰优良品种具有重要意义。本研究利用60Co-γ辐射诱变籼稻N142, 获得叶片早衰突变体 ospls3, 其叶片早衰始于分蘖期, 最先表现为叶尖变褐及叶中上部出现褐色斑点, 并向叶基部蔓延而使叶片枯死。生理分析表明, 野生型剑叶的叶绿素含量显著低于倒二叶和倒三叶, 而突变体的含量则分别低于野生型且依次显著降低; 野生型剑叶、倒二叶和倒三叶间的超氧化物歧化酶(SOD)活性、过氧化物酶(POD)活性、丙二醛(MDA)含量、O2܋含量和H2O2含量基本不变, 而突变体的这些活性和含量则依次显著升高; 野生型剑叶、倒二叶和倒三叶的可溶性蛋白含量和过氧化氢酶(CAT)活性变化不显著, 而突变体则依次降低。遗传分析表明, ospls3突变性状受1对隐性基因控制。借助图位克隆技术, 将该基因定位于第12染色体长臂的RM6953与RM28753之间, 物理距离为294 kb, 该结果为进一步克隆 OsPLS3基因并研究其功能奠定了基础。
Leaf senescence is the final stage of leaf development. However, premature aging of functional leaves leads to yield reduction and quality decline. Thus, it is very important for developing novel crop germplasms with delayed leaf-senescence characteristics through investigating the molecular mechanism of leaf senescence. In this study, an ospls3 ( Oryza sativa precocious leaf senescence 3) mutant, produced by60Co γ-radiation treatment of indica cultivar N142, was identified. The symptoms of the premature senescence mutant presented firstly at tillering stage showing brown leaf tip and brown spots in top part of leaf blade, then spread rapidly to basal part of leaf blade and led leaf to die. The physiological analysis indicated that, in the ospls3 mutant, the content of chlorophyll was the highest in the flag leaf, the following was in second-top and third-top leaves, but all of them were significantly lower than those in the wild type. The contents of MDA, O2܋, and H2O2 and the activities of SOD and POD among the top three leaves in the wild type maintained similar levels, which were significantly lower than those in the mutant. The soluble protein contents and the activity of CAT had no significant difference among top three leaves in the wild type while significantly decreased in the mutant. Genetic analysis verified that the ospls3 is a recessive mutant and was mapped in a 294 kb interval between RM6953 and RM28753 on the long arm of chromosome 12, which establishes a solid foundation for further cloning and functional studies of this gene.
叶片衰老是植物生长发育的必经阶段, 也是植物适应环境的一种重要表现[1, 2]。水稻等主要农作物在灌浆中后期功能叶早衰, 将导致其结实率显著降低、空秕率增加[3]、产量下降及品质性状(垩白和整精米率等)变劣等“ 负面” 效应[4, 5]。据报道, 水稻灌浆后期功能叶每推迟1 d衰老, 理论上可增产2%左右, 实际能增产1%左右[6], 而且还能改善稻米品质[7]。
关于植物叶片衰老成因, 现已提出许多重要的学术观点或理论假说, 包括自由基损伤说、基因调控说、光碳失衡说、营养胁迫说和激素平衡说等[8]。尽管这些假说都在一定程度上解释了叶片的衰老启动及衰老进程, 但还未真正探明植物叶片衰老的机制。利用化学诱变及转基因T-DNA插入技术获得叶片早衰突变体是研究叶片早衰分子机制的重要手段之一。基于突变体策略, 至今已从水稻中克隆了14个叶片早衰基因, 包括UAP1[9]、OsNAP[10]、OsSIK2[11]、OsWRKY42[12]、OsABC1-2[13]、SGR[14, 15]、OsAkα Gal[16]、RLS1[17]、nol1[18]、nyc1[19]、NYC3[20]、NYC4[21]、SPL28[22]和OsLMS[23]。
本课题组利用60Co-γ 辐射诱变中籼恢复系N142, 获得一个隐性叶片早衰突变体, 暂命名为ospls3 (Oryza sativa precocious leaf senescence 3)。该突变体叶片早衰表型在水稻分蘖期(约4~5片叶龄时)就开始显现, 之后随着叶龄的增加, 除心叶外, 其他叶片均不同程度早衰; 孕穗后期后则所有叶片均早衰。本文研究突变体ospls3的基本表型、生理变化及基因定位, 以期为进一步克隆该基因并揭示其早衰的分子和生理机制提供理论基础。
叶片早衰突变体ospls3, 经杭州和海南连续8代回交和自交, 获得突变性状稳定株系。之后以ospls3为母本, 分别与N142和粳稻02428杂交获得F2群体并用于遗传分析。所有材料均种植于浙江大学农业试验站。水稻成熟后, 分别取ospls3及其野生型对照N142各20株, 调查它们的株高、有效穗数、每穗粒数、结实率、千粒重和单株产量等主要农艺性状。
选取ospls3及其野生型对照N142各12株处于孕穗期(剑叶叶枕与倒二叶叶枕平齐的分蘖)的剑叶、倒二叶和倒三叶分别测定叶绿素含量、可溶性蛋白含量、H2O2含量、超氧阴离子含量(O2܋)、MDA含量、CAT活性、SOD活性和POD活性。用碧云天过氧化氢检测试剂盒(s0038)测定H2O2含量, 提取方法参见说明书。参考《植物生理学实验技术》测定其他生理指标[24], 每个指标测定3个重复。
在大田分单株剪取2014年ospls3/02428的F2定位群体中具有突变体性状的早衰单株、双亲以及F2群体中10株正常植株的叶片, 采用CTAB法[25]提取基因组总DNA。利用均匀分布于水稻12条染色体的500对SSR标记, 其引物序列来自Gramene数据库(http://www.gramene.org/), 以及Shen等[26]所开发的50对InDel标记进行基因定位。PCR总反应体系为20 μ L, 含0.8 U Taq DNA聚合酶、1 × PCR buffer (Mg2+ Plus)、1 mmol L-1 dNTP Mixture、50 ng DNA、上下游引物各0.25 μ mol L-1。PCR反应条件为95℃预变性5 min; 94℃, 30 s; 55℃, 30 s; 72℃, 30 s, 共35个循环; 72℃, 10 min。PCR产物经8%非变性聚丙烯酰胺凝胶电泳, 快速银染后观察[27]。
从786株的ospls3/02428 F2群体中获得190个具有早衰性状的单株组成基因定位群体, 利用上述筛选到的基因连锁分子标记进行基因定位。同时在Gramene数据库查找基因连锁分子标记在染色体上的具体物理位置以确定其排列顺序, 结合每个标记的重组交换单株确定OsPLS3基因与连锁标记的顺序, 构建基因定位图谱。
突变体ospls3的叶片在分蘖期就表现早衰性状(图1-A), 除心叶外, 各叶位叶片完整展开5~7 d后, 从叶尖和叶中上部开始出现褐色斑点(图1-D), 并向叶基部迅速蔓延而使叶片枯死。孕穗后期所有叶片均不同程度早衰(图1-B, C)。叶片早衰导致突变体ospls3的主要农艺性状, 如株高、有效穗数、每穗粒数、结实率、千粒重和单株产量分别比野生型下降27.74%、32.03%、69.08%、71.35%、36.03%和95.12% (表1)。
| 表1 突变体ospls3及其野生型的农艺性状 Table 1 Agronomic traits of ospls3 and its wild-type (WT) plants |
2.2.1 叶绿素含量 孕穗期突变体ospls3的剑叶、倒二叶和倒三叶的叶绿素含量及叶绿素a/b的值依次显著下降(表2), 倒二叶和倒三叶的总叶绿素含量分别比剑叶下降7.02%和53.24%。与野生型相比, 突变体ospls3的剑叶、倒二叶和倒三叶的总叶绿素含量分别下降22.78%、47.63%和74.66% (表2)。
| 表2 孕穗期突变体ospls3及其野生型叶片的叶绿素含量 Table 2 Chlorophyll content of leaves in ospls3 and its wild-type (WT) plants at booting stage |
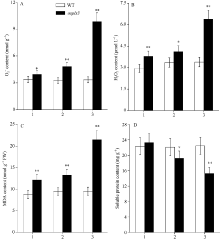
2.2.2 SOD、POD和CAT的活性 图2表明突变体倒二叶和倒三叶的SOD和POD活性显著高于野生型, 而CAT的活性则极显著低于野生型。相对于突变体剑叶, 倒二叶和倒三叶的SOD活性分别增加12.03%和21.79%, POD活性分别升高100.00%和100.76%, 而CAT活性则分别下降23.04%和47.76%。
2.2.3 超氧阴离子(O2܋)含量和H2O2含量 图3-A和B显示, 野生型剑叶、倒二叶和倒三叶间的O2܋含量和H2O2含量无显著性差异, 与野生型相比, 突变体ospls3剑叶、倒二叶和倒三叶的O2܋含量分别增加15.88%、49.08%和194.30%, 而H2O2含量则分别增加28.46%、22.79%和88.18%。
2.2.4 MDA和可溶性蛋白含量 图3-C显示, 野生型的剑叶、倒二叶和倒三叶间的MDA含量和可溶性蛋白含量无显著性差异, 与野生型相比, 突变体ospls3剑叶、倒二叶和倒三叶的MDA含量分别增加38.25%、39.96%和125.31% (图3-C); 而突变体除剑叶外, 其倒二叶和倒三叶的可溶性蛋白含量分别下降13.17%和31.83% (图3-D)。
ospls3/N142和ospls3/02428两个杂交F1单株的叶色正常, 说明突变体叶片早衰性状是由隐性位点控制。在ospls3/N142的638个F2单株中, 有153株表现叶片早衰, 485株表现正常, 正常单株∶ 早衰单株=3∶ 1 [χ 2=0.30 < 3.84 (χ 2(0.05, 1))]; 而在ospls3/ 02428的786个F2定位群体中, 正常单株为596株, 早衰单株为190株, 正常单株∶ 早衰单株 = 3∶ 1 [χ 2=0.24 < 3.84 (χ 2(0.05, 1))]。遗传结果均符合孟德尔单基因隐性遗传的分离比例, 从而证实突变体ospls3的叶片早衰症状受单隐性核基因控制。
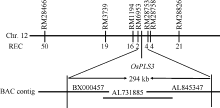
为了定位OsPLS3基因, 分别选取ospls/02428 F2群体中10株正常株和10株早衰突变株构建正常基因池和突变基因池, 选取均匀分布于水稻12条染色体上的SSR标记与InDel标记, 确定各标记与OsPLS3基因的连锁关系。结果发现水稻第12条染色体上的SSR标记RM28466、RM3739、RM1194及RM28826与OsPLS3连锁。利用这4个标记对ospls3/02428的F2 群体中的190个早衰单株进行基因型分析, 发现RM28466、RM3739、RM1194和RM28826的交换单株分别是50、19、16和21株。进一步在RM1194和RM28826之间设计了16个SSR标记, 利用其中10个在定位群体亲本间具多态性的标记将OsPLS3基因定位在RM6953和RM28753之间, 物理距离为294 kb, 横跨BX000457、AL731885和AL845347三个BAC (图4)。其间有EST支持的开放阅读框(ORF)为21个(http:// rapdb.dna.affrc.go.jp/viewer/gbrowse/build5/) (表3)。
| 表3 定位区间内的基因及功能注释 Table 3 Gene names and their annotations in the target interval |
叶片衰老最明显的外在表现是叶片黄化, 而内在生理生化变化则包括叶绿体降解、蛋白水解酶活性增加、蛋白质降解、活性氧清除系统受到抑制、自由基含量及丙二醛(MDA)含量急剧增加等[1, 28]。本研究利用辐射诱变获得叶片早衰突变体ospls3, 其叶片早衰症状始于分蘖期(图1-A), 孕穗后期则所有叶片均早衰(图1-B)。相对于野生型, 突变体ospls3的剑叶、倒二叶和倒三叶的总叶绿素含量分别下降22.78%、47.63%和74.66% (表2)。说明突变体的剑叶、倒二叶和倒三叶均已衰老, 且衰老程度依次加重。研究表明, 叶绿素a/b的值是衡量植物叶片光合效率的重要生理指标之一[29]。本研究中与突变体剑叶相比, 其倒二叶和倒三叶的叶绿素a含量下降率均比叶绿素b高(表2)。结果说明突变体叶片的光合效率将受到抑制, 且叶绿素a比叶绿素b下降更快, 究其原因, 可能是倒二叶和倒三叶中的活性氧离子含量显著高于剑叶(图3-A), 导致叶绿素a对活性氧离子的反应比叶绿素b更敏感[2, 30]。
叶绿体是植物ROS (reactive oxygen species, ROS)形成的主要场所, 伴随衰老叶片叶绿体的解体以及其他逆境胁迫, 叶绿体中的电子传递链将受到抑制, 致使1O2、H2O2和O2܋大量形成[31]。同时, 高浓度的ROS不仅作用于脂质, 使其发生过氧化反应而产生大量MDA, 加剧膜的损伤, 还通过细胞氧化应激反应诱导细胞凋亡甚至坏死[32]。本研究中野生型剑叶、倒二叶和倒三叶间的H2O2、O2܋和MDA含量无显著差异, 而突变体的含量分别显著高于野生型并依次增加(图3)。此外, 蛋白质含量特别是可溶性蛋白质含量的下降是衡量叶片衰老的重要指标之一[1]。本研究中野生型倒二叶和倒三叶的可溶性蛋白含量极显著高于突变体, 说明突变体倒二叶和倒三叶明显衰老。作为可溶性蛋白的抗氧化系统酶SOD、POD和CAT, 担负着清除细胞内ROS并保护膜系统的重任。野生型剑叶的CAT活性低于突变体(图-C), 其原因可能是野生型剑叶尚未发育完全, 前人研究也显示叶片完全发育前其CAT活性持续增加[33], 而对于突变体来说, 要及时清除过多的H2O2, 势必增加CAT活性。而突变体的CAT活性依次显著下降(图2-C), 致使细胞中H2O2不能及时被清除而累积(图3-B)。至于突变体剑叶、倒二叶和倒三叶的SOD和POD活性显著高于野生型且依次显著升高的原因, 可能是叶片在衰老初期的自我保护, 诱导了SOD和POD合成相关基因的表达(图2-A和B), 该结果进一步证实了汪媛[34]和赵晨晨等[35]的研究。当然, 叶片中高浓度的H2O2和O2܋也将导致突变体倒二叶和倒三叶中的MDA含量显著升高, 分别比剑叶增加8.29%和76.93% (图3-C)。
叶片衰老既受生物和非生物等因素的影响, 也受植物内源激素的调节。研究已证实, 乙烯、油菜素甾醇、脱落酸(ABA)、水杨酸、茉莉酸及其衍生物茉莉酸甲酯等均是叶片衰老促进激素[36]。其中ABA合成的关键限速步骤是9-顺-环氧类胡萝卜素(9-cis-epoxycarotenoids)被裂解成C15的ABA前体黄质醛, 此步骤涉及的酶是9-顺-环氧类胡萝卜素双加氧酶(9-cis-epoxycarotenoid dioxygenase, NCED)[36]。一旦内源ABA水平增加, 将诱导NAC类转录因子如AtNAP[37]和OsNAP[10]的表达, 并进一步促进其靶基因SAG113的表达[38], 进而抑制叶片气孔关闭, 加快叶肉细胞呼吸及水分蒸发, 最终导致叶片衰老。此外, 叶片衰老还受其他衰老相关基因的精细调控, 其中水稻DNA结合蛋白OsWRKY42可以与OsMT1d的启动子区域结合, 进而抑制其表达并诱导ROS反应, 促进叶片衰老[12]; 抑制水稻锌指蛋白基因OsDOS[39]或OsTZF1[40]的表达则会引起水稻叶片早衰; 过量表达水稻GTP酶基因OsRab7B3也导致叶片早衰[41]。在本研究的OsPLS3基因定位区间内, 尚没有衰老相关的基因被定位或克隆, 而与报道相关的导致水稻叶片衰老的基因分别为锌指蛋白基因(LOC_ Os12g42250)、9-顺-环氧类胡萝卜素双加氧酶基因(LOC_Os12g42280)、GTP酶基因(LOC_Os12g42370)和DNA结合蛋白基因(LOC_Os12g42420)。研究表明锌指蛋白转录因子[42]及DNA结合因子[43]可以通过与清除ROS的CAT互作, 调控叶肉细胞的ROS反应来控制叶片衰老。本研究突变体的MDA含量、CAT活性、O2܋含量、H2O2含量等结果表明叶片衰老很可能与ROS反应密切相关, 因此初步推测候选基因很可能是锌指蛋白基因(LOC_Os12g42250)或DNA结合蛋白基因(LOC_Os12g42420)。当然, 候选基因的最终确定要依赖于这两个基因的测序分析及遗传互补验证。
ospls3是一个新的叶片早衰突变体。与野生型N142相比, 孕穗期突变体叶片中的叶绿素总量显著下降。伴随ospls3叶绿体的降解, 致使叶片中的O2܋和H2O2大量产生。同时, 由于ospls3的CAT活性显著下降, 清除H2O2的能力也显著下降, 并进一步引起MDA含量的极显著增加; 然而, 在叶片衰老的初期, SOD和POD活性显著升高。该突变受1对核隐性基因控制, 定位于第12染色体SSR标记RM6953和RM28753之间约294 kb的范围内。
The authors have declared that no competing interests exist.
作者已声明无竞争性利益关系。The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|