第一作者联系方式: E-mail: 1409882837@qq.com
通过EMS (ethane methyl sulfonate)诱变籼稻品种IR64获得一个稳定遗传的显性斑点叶突变体 HM113。在大田环境下, 突变体褐色斑点在播种后3周的叶片上产生, 始穗期扩散至叶鞘。与野生型IR64相比, 突变体 HM113的株高、结实率和千粒重等农艺性状显著下降, 光合色素含量、净光合速率和可溶性蛋白含量显著降低。同时突变体CAT和SOD活性显著降低, POD活性显著上升。组织化学分析显示, 突变体叶片中积累了大量活性氧, 且斑点处细胞坏死。白叶枯病菌接种结果显示, HM113是一个广谱抗性增强的突变体。实时定量PCR分析表明 HM113中防卫反应基因 AOS2、 PAL4、 PR10和 PR1b等的表达大幅上调。遗传分析表明, 突变体褐斑性状受单显性基因( SplHM113)控制, 利用图位克隆法将该基因定位在第7染色体长臂RM21605和RM418之间, 物理距离约为308 kb。本研究为褐斑基因 SplHM113的克隆与功能分析奠定了基础。
A stable inherited rice spotted-leaf mutant HM113 was isolated from an EMS-induced IR64 mutant bank. Under natural conditions, brown lesions were observed on the leaves in three weeks after sowing and spread to the sheaths at the initial heading stage. Agronomic traits including the plant height, panicle length, number of panicles, number of filled grain/panicle, seed-setting rate and 1000-grain weight were decreased significantly in HM113. In addition, the photosynthetic pigment contents, net photosynthetic rate and soluble protein content in the mutant were significantly lower than those in the wild type IR64, while the MDA content was similar to that in the wild-type. Activities of CAT and SOD were significantly lower and activity of POD was significantly higher in the mutant than in IR64. Histochemical analysis showed that cell death and ROS accumulation were occurred in and around the lesions in HM113. Furthermore, disease resistance to bacterial blight pathogens was significantly enhanced in the mutant in contrast to that in the wild type IR64. Expression of defense-related genes including AOS2, PAL4, PR10, and PR1b was apparently up-regulated in the mutant. Genetic analysis indicated that the mutant trait was controlled by a novel single dominant nuclear gene, tentatively termed as SplHM113, which was detected to be located in a region around 308 kb flanked by RM21605 and RM418 on the long arm of chromosome 7. The data and populations obtained in the present study would facilitate the isolation and functional analysis of SplHM113.
自然界条件下, 为抵御病原菌的侵害, 植物形成了一系列复杂的信号传导途径和抗性机制。其中最有效的是过敏性反应(hypersensitive response, HR), HR是一种程序性细胞死亡(programmed cell death, PCD)[1, 2]。在HR过程中, 细胞产生大量活性氧 (reactive oxygen species, ROS), 能引起膜功能紊乱, 致使植物遭受病原菌感染部位细胞死亡以阻止病原菌的进一步侵染。HR通常会激活病程相关蛋白(pathogenesis-related, PR)的表达, 并使植物产生系统获得性抗性(systemic acquired resistance, SAR)[1, 3]。
许多斑点叶突变体在无病原菌侵染条件下产生与HR相似的表型, 并伴随斑点部位细胞坏死, 因此这类突变体又被称为类病斑或类病变突变体。许多已经鉴定的斑点叶突变体比其野生型对病原菌的抗性有不同程度的提高, 如玉米突变体LES22对白粉病菌的抗性提高[4], 拟南芥突变体hlm1对毒性丁香假单胞杆菌番茄致病变种的抗性增强[5], 水稻斑点叶突变体HM47[6]、HM143[7]和hm197[8]对水稻白叶枯病具有广谱抗性, blm对稻瘟病具有较强的抗性[9], spl17和Spl26对白叶枯病和稻瘟病均有较强的抗性[10]。因此可利用斑点叶突变体研究植物的过敏性反应以及针对不同病原菌的抗性机制。
目前, 已对水稻中的80多份斑点叶突变体完成了遗传鉴定, 斑点性状主要受单隐性基因控制, 少数受单显性基因或双基因控制。已经克隆的19个斑点叶基因, 则分别编码不同的酶/蛋白质, 参与植物体内不同的代谢途径, 如基因sl编码细胞色素P450单加氧酶, 可催化色胺生成5’ -羟色胺[11]; OsLSD1编码锌指蛋白, 调控植物的程序性细胞死亡和愈伤组织分化[12]; RLIN1[13]和FGL[14]分别编码粪卟啉原III氧化酶与原叶绿素酸酯氧化还原酶B, 参与四吡咯代谢以及叶绿素的合成; Spl7编码一个热激转录因子, 在高温胁迫下诱导产生热激反应并形成类病斑[15]; OsSSI2编码脂肪酸脱氢酶, 参与合成脂肪酸衍生物[16]; SPL28编码网格受体蛋白的复合亚基μ 1, 参与高尔基体上有关物质的运输[17]; LMR编码AAA-ATP酶, 参与植物的过敏性反应[18]。斑点叶基因编码的产物种类众多, 广泛参与各种生理生化代谢途径, 预示着斑点形成的机制十分复杂, 加强对斑点突变体的发掘鉴定与功能的深入研究, 有利于阐明斑点叶介导的抗性分子机制, 也有助于探讨该类抗性在作物育种中的应用。
我们从EMS诱变籼稻IR64的突变体库筛选到一个褐色斑点叶突变体HM113。本文针对突变体的农艺性状、生理生化、白叶枯病抗性、基因定位等进行了研究。明确了HM113是一个广谱增强的白叶枯病抗性突变体, 该斑点叶性状受一对新的显性基因控制。本研究为该基因的克隆、功能分析和白叶枯病抗性机制的阐明奠定了基础。
籼稻品种IR64经EMS诱变获得的斑点叶突变体HM113, 经过连续多代自交, 斑点叶性状在海南陵水和浙江富阳均能稳定遗传。2014年, 在成熟期随机选取突变体和野生型各3株分别考查株高、穗长、有效穗数、每穗实粒数、结实率和千粒重, 取其平均值。
参照Feng等[6]的方法, 在分蘖盛期用宽约1 cm锡箔纸对突变体HM113剑叶的无斑部位和野生型IR64相同部位叶片遮光处理, 跟踪观察斑点的发生情况, 同时观察去除锡箔纸7 d后斑点的变化。
分蘖盛期, 取突变体和野生型相同部位叶片, 参照Arnon[19]和Wellburn[20]的方法测定叶绿素含量和类胡萝卜素含量; 赵世杰等[21]的方法测定可溶性蛋白含量(soluble protein content, SP)和丙二醛含量(malonaldehyde content, MDA)。在抽穗期, 晴天上午10:00— 11:00利用便携式光合测定仪Li-6400 (LI-COR, USA)测定突变体和野生型剑叶光合作用, 按Huang等[22]的方法设定光合参数。对每个生理指标重复测定3次, 取平均值。
取突变体和野生型分蘖盛期剑叶, 采用苔盼蓝染色法检测细胞死亡状况[23], 利用二氨基联苯(diamino benzidine, DAB)和氮蓝四唑(nitroblue tetrazolium, NBT)染色法[17]分别检测过氧化氢(H2O2)和超氧阴离子(O2܋)沉积情况, 将以上脱色后的叶片用70%的甘油封片并照相记录。
参照赵世杰等[21]的方法分别测定突变体HM113和野生型IR64在分蘖盛期剑叶中的过氧化氢酶(catalase, CAT)、超氧化物歧化酶(superoxide dismutase, SOD)、过氧化物酶(peroxidase, POD)和抗坏血酸氧化酶(ascorbate peroxidase, APX)的活性。重复测定3次, 取平均值。
选取国内外8个水稻白叶枯病菌(Xanthomonas oryzaepv. oryzae)小种的10个代表菌株, 包括菲律宾菌株PXO71 (Race 4)、PXO112 (Race 5)、PXO339 (Race 9a)、PXO347 (Race 9c)和PXO349 (Race 9b)以及中国菌株JS97-2 (I)、HB17 (II)、Zhe173 (IV)、GD1358 (V)和OS-225 (VII), 利用WF-P灭菌培养基在28℃培养箱中活化3~4 d, 再转移至新的培养基中继续培养3~4 d后, 用灭菌水稀释配制成OD600约为1.0的悬浮液。在分蘖盛期, 采用剪叶法[24]对感病对照IR24、野生型IR64和突变体HM113的全展叶接种, 以灭菌的手术剪每蘸取一次菌液仅剪去一片叶的约2 cm叶尖, 每个小种接种3个单株, 每株3~4片叶。21 d后测量病斑的长度, 取10片叶病斑长度的平均值。
为明确突变体对白叶枯病的抗性增强是否与斑点相关, 选用突变体和野生型对白叶枯病抗性存在显著差异的菌株PXO349, 接种HM113/IR64回交F2群体的184个有斑植株和65个无斑单株, 21 d后测量病斑的长度。
分蘖盛期, 参照TRIzol Reagent试剂盒(Aidlab, China)的方法分别提取HM113和IR64的剑叶总RNA, 利用ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo, Japan)试剂盒将RNA反转录为cDNA。采用SYBR Premix ExTaq II (Tli RNaseH Plus)试剂盒(TaKaRa, Japan)和Thermal Cycle Dice Real Time System (TaKaRa, Japan)进行实时定量PCR分析。以水稻Actin1为内参基因, 相关基因的特异性引物序列见表1。
| 表1 实时定量PCR引物 Table 1 Primers used in Real-time PCR |
以HM113为母本, 分别与4个正常叶色品种02428、Moroberekan、Nekken 1和CPSLO17配制杂交组合, 观察F1表型; 并利用来源于HM113/Mor oberekan、HM113/Nekken 1和HM113/CPSLO17的F2群体进行性状分离分析。此外, 利用来源于HM113/CPSLO17的2个F3分离株系进行遗传验证。
采用简易法[25]提取亲本及定位群体(HM113/ 02428, HM113/Moroberekan, HM113/CPSLO17)中无斑F2单株的DNA。用分布于12条染色体上的1014对SSR引物进行亲本间的多态性标记筛选, 将筛选到的多态性标记用于HM113/02428 F2群体的106个无斑单株的基因型鉴定, 初步确定与斑点叶基因连锁的标记。根据初定位的结果, 进一步利用HM113/Moroberekan和HM113/CPSLO17两个群体的698个F2无斑单株精细定位。其中SSR引物序列来源于Gramene数据库(http://www.gramene.org/), 引物由上海生工生物工程有限公司合成。参照Shi等[26]的PCR和产物检测方法。
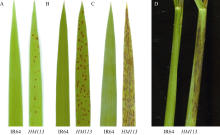
在杭州大田自然条件下, 播种后约20 d, 突变体老叶上开始出现模糊的褐色斑点, 而后斑点变大颜色变深, 逐渐连接成线状, 抽穗期扩散至整个叶片(图1-A, B, C), 始穗期叶鞘处也有褐色斑点产生(图1-D)。除始穗期比野生型晚约9 d外, 突变体的有效穗数显著低于野生型, 株高、穗长、每穗实粒数、结实率和千粒重则极显著低于野生型(表2)。而突变体株高变矮, 是由第五节间显著缩短所致(图2)。
| 表2 野生型IR64和突变体HM113的主要农艺性状表现 Table 2 Performance of agronomic traits between IR64 and HM113 |
在大田条件下为明确光照对斑点产生的影响, 对突变体和野生型叶片遮光处理。7 d后突变体遮光部位没有产生褐色斑点, 未遮光部位产生褐色斑点(图3-C); 去除锡箔纸后7 d后, 突变体原来遮光部位开始产生褐色斑点(图3-D)。表明突变体叶片褐斑的产生受自然光的诱导。
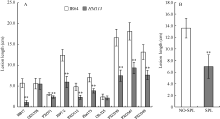
与野生型IR64相比, 突变体HM113的叶绿素a含量、叶绿素b含量、叶绿素a/b比值和类胡萝卜素含量均极显著降低(表3), 说明突变体斑点的产生可能影响了光合色素的代谢。且突变体的净光合速率(Pn)、气孔导度(Gs)、胞间CO2浓度(Ci)和蒸腾速率(Tr)极显著降低(图4-A, B, C, D), 可能与单位面积内斑点的产生减少了叶片的有效光合面积有关。HM113中SP含量比IR64极显著降低, MDA含量有所上升但并无显著差异(图4-E, F), 表明分蘖盛期斑点的产生并未引起突变体的早衰。
为检测突变体叶片斑点处的细胞是否已经死亡, 利用苔盼蓝染色法进行鉴定。结果显示野生型叶片呈均一的蓝色(图5-B), 突变体叶片斑点处呈深蓝色(图5-D), 表明突变体斑点处细胞已经死亡。活性氧(ROS)的大量累积对细胞具有明显的毒害作用, DAB和NBT能够分别与植物中的H2O2和O2܋反应, 以检测组织中是否有ROS的累积。DAB染色后, 仅突变体叶片上沉积了大量的红褐色物质(图5-H); NBT染色后, 野生型叶片有少数蓝色染斑(图5-J), 而突变体叶片产生了大量蓝色染斑(图5-L), 说明活性氧H2O2和O2܋的积累导致了突变体叶片的死亡。
正常植物体内活性氧(ROS)的产生与清除处于动态平衡中, 若该平衡遭到破坏, 就会引起ROS的大量累积并对细胞造成氧胁迫, 引起细胞损伤甚至死亡。为探究突变体中积累大量ROS的机制, 测定了分蘖盛期叶片中抗氧化系统重要酶的活性。与野生型相比, 突变体的CAT活性极显著降低, SOD活性显著降低, POD活性极显著上升, 而APX活性没有显著变化(图6), 以上结果表明突变体的活性氧清除系统遭到破坏。
| 表3 野生型IR64和突变体HM113的光合色素含量 Table 3 Photosynthetic pigment contents of IR64 and HM113 |
与野生型相比, 突变体对菌株HB17、PXO71、JS97-2、PXO112、Zhe173、PXO339、PXO347和PXO349抗性极显著增强; 对菌株GD1358和OS-225的抗性则无明显差异(图7-A)。选用菌株PXO349接种HM113/IR64回交F2群体, 其中184个有斑植株的病斑平均长度为6.96 cm ± 2.04 cm, 极显著低于65个无斑植株的13.58 cm ± 1.07 cm (图7-B)。说明突变体对白叶枯病菌的抗性普遍增强, 且抗性增强与褐斑的存在高度相关。
为了解突变体对白叶枯病的抗性机制, 利用实时定量PCR检测突变体和野生型中6个防卫反应基因的表达, 这些基因分别编码丙二烯氧化物合酶2 (alleneoxide synthase 2, AOS2)、脂氧合酶(lipoxygenase, LOX)、苯丙氨酸解氨酶(phenylalanin ammonia lyase, PAL4)、病程相关蛋白(pathogenesis- related protein) PR10和PR1b以及病程相关因子NPR1的同源物(NPR1 homolog, NH1)。结果表明, 突变体中这6个基因的表达水平均比野生型极显著上升(图8), 说明褐斑的产生激活了突变体相关防卫反应基因的表达。
来源于HM113/02428、HM113/Moroberekan、HM113/Nekken 1和HM113/CPSLO17组合的所有F1植株均呈现褐色斑点表型, 但褐斑的数量较突变体少, 说明突变体的褐斑性状受显性基因控制, 但存在剂量效应(也有学者称为半显性)。HM113/Morob erekan、HM113/Nekken 1和HM113/CPSLO17的F2群体的有斑单株与无斑单株数均符合3∶ 1 (χ 20.05= 3.84)的分离比(表4), 来源于HM113/CPSLO17的2个F3分离株系的有斑单株数与无斑单株数的分离比也都符合3∶ 1 (数据未发表)。说明突变体褐斑性状受单显性基因控制, 暂将该基因命名为SplHM113。
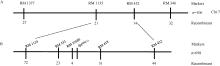
为定位该显性基因, 挑选出均匀分布于12条染色体上HM113和02428间的多态性SSR标记139对, 用HM113/02428的F2群体的106个无斑单株初定位, 将斑点叶基因定位在第7染色体RM1135和RM432之间。进一步利用来自HM113/Moroberekan和HM113/CPSLO17 F2群体的698个无斑植株进行精细定位, 将基因SplHM113定位在RM21605和RM418之间, 物理距离约308 kb (图9)。
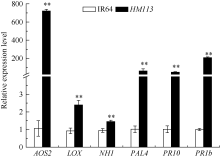 | 图8 突变体HM113中防卫反应基因的表达分析 * * 极显著差异(P ≤ 0.01)。Fig. 8 Expression level of defense-related genes in HM113 * * Significantly different at P ≤ 0.01. |
| 表4 斑点叶突变体HM113的遗传分析 Table 4 Genetic analysis of HM113 |
自第一个水稻斑点叶突变体被报道以来, 越来越多的水稻斑点叶突变体得到鉴定。斑点叶突变体在无明显逆境或病原菌侵染条件下, 在叶片上自发产生斑点, 并伴随农艺性状的改变, 如spl31[27]、spl21[28]和lmm6[29]。本研究的突变体HM113播种后20 d左右, 老叶上出现褐色斑点, 抽穗期扩散至整个叶片, 同时叶鞘处也有褐色斑点产生。与IR64相比, HM113的植株变矮, 穗变短, 有效穗数和每穗实粒数减少, 结实率和千粒重下降。坏死性斑点的产生降低了叶片中单位面积的光合色素含量、气孔导度和胞间CO2浓度, 导致突变体净光合速率降低, 进而影响突变体的农艺性状。
ROS是植物细胞内部氧化还原反应的产物。正常情况下, 植物内部多余的ROS能够被抗氧化剂及时清除; 在胁迫环境下, 当植物细胞内的H2O2浓度瞬时达到1 mol L-1时, 活性氧清除系统就会遭到破坏[30]。活性氧清除系统包括CAT、SOD和POD等。与CAT和SOD的清除活性氧的功能不同, POD是一种多功能酶, 既能协助清除叶绿体中H2O2[31], 又能直接或间接催化形成H2O2和O2܋, 研究报道细胞壁上的POD参与了植物体内ROS迸发[32, 33, 34]。本研究中, 突变体HM113叶片中活性氧的瞬时大量产生, 以及CAT和SOD活性显著降低, POD活性显著上升可能是导致叶片产生大量ROS的主要原因。
许多水稻斑点叶突变体对白叶枯病或稻瘟病具有较强的抗性。加强对斑点叶突变体的研究有利于揭示植物的抗性机制。如spl28对白叶枯病和稻瘟病的抗性增强可能与防卫反应基因PR1、PR2的激活以及胼胝质、酚类物质和植物抗毒素的累积有关[17]; 类病变突变体spl5对白叶枯病抗性增强与接种后的保护酶POD和PAL活性提高、OsPR1和OsPR8基因表达水平提高具有紧密的联系[35]。研究报道ROS大量累积会影响植物的水杨酸(SA)或茉莉酸(JA)抗性途径, 增强对病原菌的抗性; 此外, 引起的氧化胁迫对细胞具有毒害作用, 引起细胞死亡, 并使植物产生获得性免疫抗性[36, 37]。本研究中, 突变体HM113对8个水稻白叶枯病菌小种的抗性均明显增强, 该抗性与褐斑的产生高度相关。推测褐斑发生过程中, 叶片中积累了大量的H2O2和O2܋, 引起褐斑部位及其周围细胞坏死, 阻止了病原菌的进一步侵染; 同时激活防卫反应基因AOS2、PAL4、PR10和PR1b等的表达, 提高了突变体的抗病性。
迄今为止, 水稻中已经鉴定了10个显性斑点叶突变体, 其中仅NH1[38]、OsAT1[39]和Spl32(t)[40] 3个显性斑点叶基因被鉴定, 分别位于第1、第10和第11染色体。NH1是与拟南芥NPR1同源的病程相关因子, 能拮抗调控SA和JA响应基因, NH1的过表达能提高植株对白叶枯病的抗性[41, 42]; OsAT1编码一个酰基转移酶, 其突变体中PR基因表达上调, 植保素(稻壳酮和樱花素)含量升高, 对稻瘟病的抗性增强[39]。目前在第7染色体上鉴定了3个斑点叶基因, 其中lems1[43]位于长臂RM1364和RM420之间, spl5[44]位于短臂SSR7和RM7121之间, splNF4050-8[45]位于短臂NBARC1和RM8262之间, 可能与spl5等位或紧密连锁, 这些斑点性状均由单隐性基因控制。本研究的斑点叶基因SplHM113被定位于第7染色体长臂RM21605和RM418之间, 该区间内未有显性斑点叶基因的报道, 因此SplHM113是一个新的斑点叶基因。
斑点叶突变体HM113褐色斑点的产生受光照的诱导并影响光合色素含量、光合作用以及主要农艺性状。突变体的ROS清除系统的失衡包括CAT和SOD活性显著降低及POD活性显著上升, 可能是引起ROS沉积与细胞坏死的主要原因。此外, 突变体HM113对白叶枯病抗性的显著增强可能与ROS的累积及防卫反应基因的激活表达有关。新型显性斑点叶基因SplHM113的鉴定, 为该基因的克隆与功能研究奠定了基础, 有利于进一步解析类病斑介导的植物抗性机制和探讨该类型抗性在作物育种中的应用前景。
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|