第一作者联系方式: 闫蕾, E-mail: yanlei2723@126.com, Tel: 010-82105851; 杨宗举, E-mail: zongjuyang@163.com
隐花色素(cryptochrome, CRY)是植物蓝光的主要受体, 参与其调节生长发育及生物钟过程。为研究隐花色素在玉米光形态建成及生物钟调控方面的作用, 本研究利用同源克隆的方法得到玉米自交系B73的2个 ZmCRY1a基因的cDNA序列, 分别命名为 ZmCRY1a1和 ZmCRY1a2。这2个基因的编码区(coding DNA sequence, CDS)序列长度都为2124个核苷酸, 编码707个氨基酸。生物信息学分析表明ZmCRY1a1和ZmCRY1a2推测的氨基酸序列均包含DNA photolyase、FAD binding和Crytochrome C结构域; 与拟南芥及其他常见作物的CRY比对并构建系统发育树显示, 这2个基因与水稻OsCRY1a氨基酸序列一致性最高, 而与拟南芥和大豆等双子叶植物的CRY1氨基酸序列一致性相对较低。利用实时荧光定量PCR分析了 ZmCRY1a1和 ZmCRY1a2在不同器官及响应光质、光质转换及长日照与短日照处理的表达模式。在检测的器官中, ZmCRY1a1的表达丰度均高于 ZmCRY1a2; 这2个基因在成株期叶片中表达丰度最高, 分别是根中 ZmCRY1a1的52.1倍和6.2倍。相对于黑暗下, 二者在各种持续光质中的表达丰度均较高, 尤其在蓝光和远红光条件下。尽管是作为编码蓝光受体的基因, 2个 ZmCRY1a的表达却能强烈地响应远红光和红光转换处理。同样二者也能响应不同光周期处理, 长日照条件下, ZmCRY1a1的转录在一个光周期内共出现5个峰值, 而 ZmCRY1a2的转录只有4个峰值; 短日照条件下, 2个 ZmCRY1a的表达出现了极其相似的模式, 均在进入黑暗后10 h和14 h时出现2个最高峰。由此推测2个 ZmCRY1a可能在玉米光形态建成与开花调节中发挥重要作用。
Cryptochromes are blue light receptors that regulate the development of growth and circadian clock in plants. To stress the functions of crytochrome 1 ( CRY1) on photomorphogenesis and flowering regulation in maize ( Zea mays L.), we isolated the cDNA clones of two ZmCRY1a genes from inbred line B73 by homologous cloning, and designated as ZmCRY1a1 and ZmCRY1a2. The length of both ZmCRY1a coding DNA sequences were 2124 nucleotides, which encoded 707 amino acid residues. Bioinformatics analyses were employed to predict their function domains and to build a phylogenetic relationship tree among plant CRY1 homologs by the DNAMAN software and the NCBI blast. The two ZmCRY1a proteins possessed three function domains: DNA photolyase, FAD binding, and crytochrome C domains. Phylogenetic analysis indicated that the two ZmCRY1a proteins belonged to the same branch with OsCRY1a, while showing low similarity to other CRY1 proteins from dicotyledonous species, such as A. thaliana and Glycine max. The transcription abundances of two ZmCRY1a genes in different organs and in response to light treatments were detected using quantitative RT-PCR (qRT-PCR). qRT-PCR assays indicated that the two ZmCRY1a genes were highly expressed in leaf with 52.1 or 6.2 times higher than ZmCRY1a1 abundance in root, respectively. The transcription abundances of the both genes were very high under different continuous light conditions, especially in blue and far-red light. Although encoding blue light receptors, they both greatly responded to dark-to-far-red and dark-to-red transitions. In addition, their transcription abundances could also respond to photoperiod treatment (both long-day and short-day conditions). In long-day condition, The transcription abundances of ZmCRY1a1and ZmCRY1a2 had five four peaks, respectively. In short-day condition, both ZmCRY1a genes had two big peaks which happened at 10 h and 14 h after transition into darkness. Our results suggest that both ZmCRY1a genes may be involved in seedling de-etiolation and flowering time control, thus their roles in crop improvement are worthy of more exploration in the future.
玉米(Zea may L.)不但是重要的粮食作物, 而且是高光效的C4模式植物。高等植物主要的蓝光受体隐花色素(cryptochrome, CRY)参与光形态建成、开花诱导、种子休眠和生物总量等性状的发育过程。光是影响植物生长发育的关键因素之一, 既作为供给植物光合作用的能量物质, 又以信号物质参与调节植物生长发育, 包括种子萌发、茎节伸长、向光性、避荫性(shade avoidance)和开花期, 以及作物的产量和品质等[1, 2, 3, 4, 5]。植物通过光受体来感知周围环境中光线成分、强弱及节律的变化。
隐花色素是一类在高等真核生物中广泛存在的黄素类蛋白, 主要负责吸收蓝光(400~500 nm)和紫外光-A (UV-A; 315~400 nm)[6]。作为一类重要的光受体, 隐花色素不但负责调节植物的生长和发育, 并且还参与包括植物和动物的生物钟调控[7, 8, 9, 10]。植物至少含有CRY1和CRY2两类隐花色素, 其中CRY1主要调节高辐照强度蓝光下的去黄化反应; 而CRY2则是低辐照强度蓝光的主要受体, 参与光周期介导的开花调节[7, 11]。隐花色素蛋白有2个结构域, N端结构域与光解合酶活性相关(photolyase-related, PHR), 负责吸收光量子。C端结构域为核/质运输和蛋白质间相互作用所必须[11, 12]。拟南芥的CRY1蛋白属于蓝光稳定型, 而拟南芥黄化幼苗中的CRY2蛋白转换到蓝光条件下会被迅速磷酸化并降解[13, 14, 15, 16]。拟南芥的隐花色素家族还包括第3个成员CRY3, 这种仅在叶绿体和线粒体中检测到的CRY-DASH蛋白尽管缺少CRY蛋白典型的C末端的延伸, 但仍可能具有单链DNA光裂合酶活性[17, 18, 19]。拟南芥CRY1蛋白主要在细胞核与细胞质之间穿梭并发挥作用, 而翻译后的CRY2蛋白仅在细胞核中完成生活周期和生理功能[15]。
在真菌[20]、苔藓[21]、蕨类[22]、番茄[23, 24]、甘蓝型油菜[25]、豌豆[26, 27]、大豆[28, 29]和水稻[30, 31]中隐花色素基因的研究取得了不少实质性的进展。与拟南芥中功能类似, 隐花色素在多种被子植物中作为光受体和参与生物钟调控。甘蓝型油菜BnCRY1的转录能被蓝光所诱导[25]; 拟南芥、番茄和豌豆的CRY1的表达丰度也受到生物钟调控[25, 32, 33]。植物的隐花色素在开花诱导中起重要作用。拟南芥的CRY2在蓝光下通过抑制光周期调节开花诱导重要组分CONSTANS (CO)蛋白的降解而促进开花诱导基因FLOWERING LOCUS T (FT)的表达, 从而促进开花[11]。CRY2也可以通过与CIB1 (cryptochrome interacting basic helix loop helix, 隐花色素相互作用bHLH1)蛋白互作, 来激活FT的表达, 同样促进开花[34]。对作物大豆隐花色素功能研究取得突破性进展, 受光周期节律调节的GmCRY1a蛋白水平和光周期诱导的开花与品种的纬度分布紧密相关, 因此, GmCRY1a是大豆光周期诱导开花期的主要调节因子[28]。
隐花色素与植物的抗逆也密切相关。光激活的大豆GmCRY2a通过与CIB1的直接互作, 阻止其激活衰老相关基因如WRKY DNA BINDING PROTEIN53b (WRKY53b)的表达, 进而抑制大豆的叶片衰老[29]。对转小麦TaCRY1拟南芥的研究发现, 转基因植株通过调节胁迫与ABA响应基因的转录, 来增强对植物高盐、高渗透势和ABA的抗性[35]。最近研究表明, 蓝光下大麦中CRY1通过提高ABA的含量来抑制休眠种子萌发[36]。同样也发现甘蓝型油菜BnCRY1响应ABA和甘露醇处理, 并且激活生物和非生物胁迫的信号通路[37]。
模式植物拟南芥的隐花色素研究已经较为深入, 如对水稻、小麦、高粱、大麦CRY1的研究, 但对玉米CRY1a (ZmCRY1a)一直未见报道。本研究通过分析玉米ZmCRY1a在不同器官、各种光处理下的表达丰度, 明确玉米ZmCRY1a响应不同光质处理的模式, 不但丰富植物隐花色素基因功能研究, 而且为利用玉米隐花色素途径进行品种改良奠定基础。
玉米自交系B73由李新海博士惠赠、由杨建平实验室繁育并保存。
1.1.1 器官特异性表达样品 自然条件下生长60 d后分别取成株的叶、茎、根、幼穗、苞叶、花丝、雄花、花柄、叶鞘和叶枕。
1.1.2 各种持续光质处理 玉米自交系B73在22℃, 黑暗(Dk)、远红光(FR, 1.9 µ mol m-2 s-1)、红光(R, 22.3 µ mol m-2 s-1)、蓝光(B, 13.0 µ mol m-2 s-1)和白光(WL, 17.0 µ mol m-2 s-1)培养箱中生长13 d。
1.1.3 黑暗转换各种光质 在22℃黑暗中生长13 d后分别转入以上各种光质条件如1.1.2下0.25、0.5、1、2、4、8、12和24 h, 分别取样。
1.1.4 光周期长日照和短日照处理 玉米幼苗在22℃长日照(LD, 16 h光照/8 h黑暗)或短日照(SD, 8 h光照/16 h黑暗)条件下生长13 d, 每隔2 h取样, WL和Dk互相转换时提前5 min取样。
大肠杆菌DH5α 菌株和克隆载体pEASY-Blunt- Simple分别购自北京天根公司和北京全式金生物技术有限公司。
PrimeSTAR HS酶购自TaKaRa, Revert Aid First Strand cDNA Synthesis Kit购自Thermo Scientific, 限制性内切酶和T4 DNA连接酶购自NEB公司, 凝胶回收试剂盒和质粒提取试剂盒购自天根生化科技有限公司。
以TRIzol (Invitrogen, USA)法提取玉米总RNA, 经DNase I (RNase-free, TaKaRa)处理后作为模板, 以Oligo-dT18为引物, 利用Revert Aid Frist Strand cDNA Synthesis Kit (Thermo Scientific)反转录成cDNA备用。
根据NCBI中的ZmCRY1a1 (ZM05G31560)、ZmCRY1a2 (ZM04G17060)的序列设计引物(表1), 用黑暗条件下13 d幼苗的cDNA为模板, 均分别扩增得到2124 bp的片段, 与pEASY-Blunt-Simple载体连接, 经PCR和酶切鉴定后由北京奥克鼎盛生物科技有限公司测序, 测序正确后备用。
| 表1 同源克隆所用引物 Table 1 Primers for homologous cloning |
利用NCBI-Blast网站(http://blast.ncbi.nlm.nih. gov/Blast.cgi)获得玉米(Zea maysL.)、拟南芥(Arabidopsis thaliana)、水稻(Oryza sativaL.)、高粱(Sorghum bicolor)、小麦(Triticum aestivum)、大麦(Hordeum vulgare L.)、甘蓝型油菜(Brassica napus L.)、豌豆(Pisum sativumL.)、番茄(Solanum lycopersicum)、大豆(Giycine max) CRY1推导蛋白的氨基酸序列。使用DNAMAN Version 6软件比对ZmCRY1a蛋白与其他作物氨基酸序列, 用SMART网站(http://smart.embl-heidelberg.de/)分析ZmCRY1a蛋白的结构域。
根据目的基因序列, 利用Primer Premier 5.0软件设计荧光定量PCR引物, 内参基因为Tubulin[38](表2)。荧光定量PCR仪为Roche 480 (Roche, 瑞士), PCR程序为95℃ 30 s; 95℃ 5 s, 60℃ 20 s, 72℃ 10 s, 50个循环。然后绘制60~95℃溶解曲线。采用定量PCR试剂SYBR Premix Ex Taq II (TaKaRa大连公司), 按照商家说明操作。采用2-Δ Δ CT的方法计算结果[39], 经3次独立的生物学重复, 并以此计算其标准差。
| 表2 qRT-PCR所用引物 Table 2 Primers for qRT-PCR |
根据NCBI中ZmCRY1a1和ZmCRY1a2的mRNA对应cDNA序列(ZM05G31560、ZM04G17060)设计引物, 用RT-PCR分别得到2124 bp左右的DNA片段(图1-A), 与预计扩增的2个ZmCRY1a的ORF片段大小一致。将扩增片段回收并连接到pEASY- Blunt-Simple载体上, 得到重组载体pEASY- ZmCRY1a1和pEASY-ZmCRY1a2, 经PCR鉴定后的克隆再经BamH I和Sma I双酶切验证, 符合要求的克隆经酶切得到2124 bp左右的目的基因条带及3830 bp左右的载体条带(图1-B)。对符合要求的克隆测序, 显示得到的cDNA克隆序列与NCBI中2个ZmCRY1a的mRNA对应序列(ZM05G31560和ZM04G17060)完全一致。
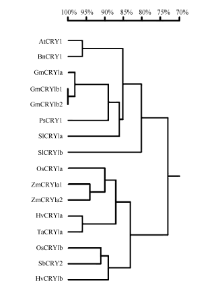
通过RT-PCR同源克隆、测序得到玉米ZmCRY1a1和ZmCRY1a2的全长cDNA序列, 其ORF包含2124个核苷酸残基, 编码707个氨基酸残基, 蛋白质分子量分别为79.9 kD和79.7 kD, 等电点分别为4.85和5.32 (http://web.expasy.org/protparam)。利用NCBI网站(http://www.ncbi.nlm.nih.gov/)和DNAMAN Version 8软件对二者的结构域分析, 并与拟南芥和水稻的CRY1进行氨基酸水平的序列比对(图2)。与其他2个CRY1结构类似, ZmCRY1a1和ZmCRY1a2蛋白均包含1个PHR结构域(1个DNA photolyase结构域和1个FAD binding 7结构域)、1个Cryptochrome C (CCE)结构域。利用DNAMAN Version 8将其氨基酸序列与拟南芥[7]、水稻[24]和大麦[36]等CRY1序列进行系统发育树分析(图3), 结果表明, ZmCRY1a1和ZmCRY1a2蛋白间氨基酸水平上的一致性为91.67%, 它们与水稻、小麦CRY1的一致性分别为90%和86%; 而与拟南芥CRY1的一致性只有70%。可见, CRY1在单、双子叶植物进化上明显的差异暗示CRY1功能可能存在分化。
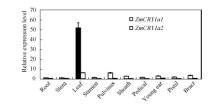
基因的表达部位与其功能关系密切, 通过qRT-PCR分析了2个ZmCRY1a在根、茎和叶片等器官中表达的差异。由于土壤中的根受光的影响较小, 把ZmCRY1a1在根中的表达丰度设为对照, 并设为1。ZmCRY1a1在茎、花丝、花柄和雄花中表达丰度与其根中ZmCRY1a1的表达丰度相似(1.1~1.7倍), 在叶鞘、幼穗、苞叶和叶枕中表达丰度稍高(根中ZmCRY1a1的2.7~6.1倍), 而叶片中表达丰度最高, 是根中ZmCRY1a1的52.1倍(图4)。ZmCRY1a2在叶中表达丰度也较高(根中ZmCRY1a1的6.2倍), 而在各种器官中的表达丰度仅为根中ZmCRY1a1的0.2~ 0.7倍(图4)。作为编码光受体的基因, 2个ZmCRY1a在玉米叶中高水平表达, 可能与其更有效地发挥作用存在密切关系。
为了研究2个ZmCRY1a基因对各种光质的响应, 采用qRT-PCR方法分析它们在黑暗(Dk)和持续远红光(FRc)、红光(Rc)、蓝光(Bc)和白光(WLc)下表达丰
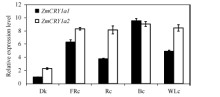
度。ZmCRY1a1在黑暗条件下的表达丰度较低, 作为对照并设为1。在黑暗条件下ZmCRY1a2的表达丰度是ZmCRY1a1的2.3倍。2个ZmCRY1a对各种光质均有较强的响应, 特别是二者在持续蓝光条件下的表达丰度最高, 均达到黑暗下ZmCRY1a1表达丰度的9倍以上。ZmCRY1a1在持续远红光下的表达丰度也较高, 是自身黑暗时的6.3倍。ZmCRY1a1在持续红光和白光下的表达丰度基本一致, 分别是自身黑暗时的3.7倍和4.9倍。与ZmCRY1a1不同, ZmCRY1a2在持续远红光、红光、蓝光和白光下基本一致, 是ZmCRY1a1黑暗时的8.2~9.2倍。持续光条件下的转录表达分析结果暗示, 2个ZmCRY1a除了参与玉米蓝光途径的调节外, 可能也在远红光和红光信号途径中起重要作用, 特别是ZmCRY1a2对各种光质均有较强的反应。
将黑暗中生长13 d的玉米自交系B73幼苗转入远红光、红光、蓝光和白光下0.25、0.5、1、2、4、8、12和24 h, 来进一步研究2个ZmCRY1a表达丰度对不同光质的响应。将ZmCRY1a1和ZmCRY1a2在黑暗条件下的表达丰度设为各自基因的对照, 并均设为1。我们首先考察了2个ZmCRY1a1对蓝光的响应(图6-A), 在由黑暗转换到蓝光8 h内, ZmCRY1a1的表达丰度持续上升至最大值(自身黑暗时的9.6倍); 之后在12 h时下降到自身黑暗时的3.2倍; 最后在24 h时其丰度又上升到自身黑暗时的6.9倍。在黑暗转换到蓝光时ZmCRY1a2表达模式与ZmCRY1a1较类似, 只是在2 h内其转录丰度并没有上调, 维持在自身黑暗时的0.05~1.40倍, 4~8 h上升
到自身黑暗时的5.0~6.3倍; 12 h时下降到自身黑暗时的2.1倍, 以后逐步上升, 在24 h时表达丰度达到自身黑暗时的24.7倍达最大值。ZmCRY1a1在由黑暗转换到白光的0.25、4、12、24 h出现4个峰值, 分别是自身黑暗时的1.4、2.2、2.8和5.1倍(图6-B)。在黑暗转换到白光时ZmCRY1a2表达模式与ZmCRY1a1较类似, 在0.25、4、12、24 h时同样出现4个峰值, 但12 h时峰值巨大(为其黑暗时的9.0倍), 其另外3个峰值也达到ZmCRY1a1对应峰值的1.6~2.3倍(图6-B)。
尽管是作为编码蓝光受体的基因, 由于这2个ZmCRY1a在持续远红光和红光中有相对高的表达丰度, 进一步考察它们对远红光和红光的响应(图6-C)。在由黑暗转换到远红光0.25 h时ZmCRY1a1的表达丰度未见明显的变化; 0.5 h时其表达丰度略下降到自身黑暗时的56%; 然后在1 h和2 h时其丰度稳步上升, 在4 h时达到第一个峰值(自身黑暗时的3.6倍); 之后在8 h时其丰度又迅速下降(自身黑暗时的1.2倍); 随后在12 h时其丰度继续下降到自身黑暗时的0.8倍; 最后, 在24 h时回升到最高峰值(自身黑暗时的4.8倍)。在黑暗转换到远红光时ZmCRY1a2表达模式与ZmCRY1a1较类似, 有3点明显的差异, 一是ZmCRY1a2表达丰度的第一个峰值发生在黑暗转换到远红光1 h时, 早于但低于ZmCRY1a1; 二是ZmCRY1a2在8 h时也形成与自身黑暗时相当的一个小峰; 三是ZmCRY1a2在24 h时达到最高峰值略低于ZmCRY1a1 (自身黑暗时的3.9倍)。最后检测了ZmCRY1a1对红光的响应, 在由黑暗转换到红光0.25 h时, ZmCRY1a1的表达丰度上升到自身黑暗时的1.7倍, 在0.5 h时迅速下降到自身黑暗时的74%; 在1~4 h, ZmCRY1a1的表达丰度持续上升, 在4 h时达到其自身黑暗时12.9倍, 随后在8 h时下降到与自身黑暗时相当水平, 之后稳步上升, 在24 h时达到自身黑暗时的22.8倍。在黑暗转换到红光时ZmCRY1a2表达模式与ZmCRY1a1类似, 但ZmCRY1a2在黑暗转换到红光1 h时出现一个小峰(是自身黑暗时的4.7倍); 在24 h时仅上升到自身黑暗时8.6倍, 远低于ZmCRY1a1。综合不同持续光质和由黑暗转换到各种光质处理下的结果可以看出, 2个ZmCRY1a表达丰度不仅响应蓝光和白光处理, 而且也能强烈地响应远红光和红光。
长日照条件下, ZmCRY1a1在光照阶段有3个明显的表达峰, 分别出现在4、8和12 h, 是自身黑暗时的2.4、3.0和4.2倍; ZmCRY1a1在进入黑暗阶段18 h和22 h时有2个明显的表达峰, 分别是自身黑暗时的2.5倍和1.7倍(图7-A)。ZmCRY1a2在长日照条件下光照阶段的3个表达峰与ZmCRY1a1趋势完全一致, 只是峰值略高, 在4、8和12 h时分别是自身黑暗时的4.0、4.4和6.6倍; ZmCRY1a2仅在进入黑暗阶段的18 h时出现一个极强峰值, 是自身黑暗时的14.9倍(图7-B)。
在短日照条件下, 2个ZmCRY1a的表达出现了极其相似的模式, 在光照阶段的2 h和8 h时出现2个小的表达峰(分别是自身黑暗时的1.1~1.7倍)(图7-C, D)。在黑暗阶段, 2个ZmCRY1a均出现1个小的和2个显著的表达峰; 在14 h时小的表达峰, 分别是其本身黑暗时的1.3倍和2.2倍(图7-C, D); 在18 h和22 h 时2个显著峰, 分别是其本身黑暗时的5~6倍。可见2个ZmCRY1a的表达能响应长日和短日处理。
隐花色素不但参与植物光形态建成[27]、开花调节[26, 32, 33]和种子休眠等发育过程, 也负责生物与非生物胁迫的调节[36, 37]。与拟南芥和水稻等普通的二倍体不同, 玉米被认为起源于一个古四倍体, 其染色体组经历基因组和片段复制、染色体融合及易位等[40, 41, 42], ZmCRY1a也保留了2个拷贝。本研究发现, 2个ZmCRY1a在玉米的叶片中表达丰度最高(图4), 同时它们的表达不但响应蓝光和白光处理, 而且响应远红光和红光(图5和图6), 以及光周期(长日照和短日照)处理(图7)等。表明它们可能参与玉米在不同光质下的光形态建成及光周期的调节。2个ZmCRY1a在进化关系上与水稻CRY1a亲缘关系最近, 而与拟南芥等双子叶植物的CRY1亲缘关系相对较远(图2和图3)。ZmCRY1a与单子叶水稻和小麦的CRY1a一致性较与双子叶拟南芥和大豆的CRY1a一致性更高(图3), 表明ZmCRY1a与同属于单子叶的CRY1具有更相似的功能。
植物通过光受体来感知光质、光强和周期的变化, 进而调节自身的生长和发育进程。近期的研究也发现甘蓝型油菜BnCRY1的表达丰度在茎、花、幼嫩长角果和根中均较高, 该基因负责调控下胚轴和茎的生长以及花青素的积累[25]。与OSCRY1a[30]类似, 2个ZmCRY1a在叶片中高表达(图4), 可能植物叶片巨大的表面积, 为光受体更好接受光线提供了便利。除在叶片中高表达外, ZmCRY1a1在其他器官中表达各异, 而ZmCRY1a2在其他各种器官中的表达丰度基本一致(图4), 推测二者在各种器官中的功能存在分工。近期的报道表明甘蓝型油菜BnCRY1基因在白光中的表达丰度远高于在黑暗[25]。2个ZmCRY1a对不同光质均存在较强的响应, 特别是对蓝光响应最强; 但是ZmCRY1a1对蓝光、远红光、红光和白光的响应存在差异, 而ZmCRY1a2基因对这些光质的响应类似, 暗示2个ZmCRY1a在各种光质下可能也存在功能的分工。另外, 2种ZmCRY1a基因非常强烈地响应黑暗转换到远红光和红光(图6), 暗示它们可能参与玉米调控远红光和红光反应。
隐花色素在蓝紫光区有3个吸收峰(420、450和480 nm), 在近紫外光370 nm左右有一个吸收峰, 而对红光(600~700 nm)和远红光(700~750 nm)没有吸收峰。拟南芥隐花色素与光敏色素的遗传与蛋白存在互作[43, 44, 45, 46, 47, 48, 49], 并且通过互作调节植物光形态建成、生物钟和种子萌发等发育过程[43, 44, 45, 46, 47, 48, 49]。是否2个ZmCRY1a可能与6个光敏色素存在蛋白互作, 在红光和远红光处理下光敏色素发挥作用并通过与隐花色素之间的互作来影响2个ZmCRY1a蛋白的稳定性, 进而反馈调节2个ZmCRY1a的表达丰度, 值得进一步探索。由于玉米光敏色素有6个拷贝, 而蓝光受体, 除了2个拷贝的ZmCRY1a以外, 还包括ZmCRY1b和ZmCRY2; 这4个蓝光受体与6个光敏色素间的互作模式, 以及在互作与活性之间的关系有待阐明。
在模式植物拟南芥中的研究表明, 高等植物开花的调控是由光信号转导途径与生物钟途径(circadian clock)相关基因如GI (GIGANTEA)、CO (CONSTANS)和FT (FLOWERING LOCUS T)共同完成的[50, 51, 52]。CRY2通过抑制CO蛋白的降解和促进FT的表达, 来促进植物开花[11, 34]。近期的研究结果显示甘蓝型油菜BnCRY1在促进开花中起作用[25], GmCRY1a与OsCRY1a也参与开花调控[28, 30]。本研究表明2个ZmCRY1a的表达强烈地响应长日照和短日照处理(图7), 2个ZmCRY1a与玉米开花调控基因ZmGI、ZmCOL和ZmFT之间的关系需进一步验证。2个ZmCRY1a对各种光质及长日照、短日照的响应, 推测其可能参与玉米光形态建成及开花调控, ZmCRY1a在玉米改良中的作用值得进一步探讨。
玉米ZmCRY1a与拟南芥AtCRY1和水稻OsCRY1a具有相似的结构, 都包含PHR结构域(DNA photolyase结构域和FAD binding 7结构域)和Cryptochrome C结构域(CCE结构域); ZmCRY1a在叶片中表达丰度最高, 并且受到不同光质的影响, 能强烈响应远红光处理, 对长日照和短日照处理也有很强的响应。推测2个ZmCRY1a在玉米光形态建成与开花调节中发挥重要作用。
The authors have declared that no competing interests exist.
作者已声明无竞争性利益关系。The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|