第一作者联系方式: E-mail: qiaoyu306@126.com, Tel: 010-62734550
缺钾诱导早衰条件下, 根-冠互作对棉花叶片早衰的调节包括地上部主导型和根系主导型。本研究主要观察缺钾条件下2种根-冠互作类型单接穗单砧木(I型)和双接穗单砧木(Y型)嫁接接穗下胚轴的解剖结构, 并判断其与下胚轴木质部中CTK和ABA的流量是否有关。结果表明, 地上部主导型I型嫁接接穗下胚轴解剖结构与砧木品种更为相似, 与其木质部CTK和ABA的流量主要受地上部调节不符; Y型嫁接2个不同品种接穗之间下胚轴解剖结构的差异与其木质部CTK和ABA流量的差异虽然具有相关性, 但可能是一种伴生现象。根系主导型I型嫁接砧木对接穗的影响很大, 但这与砧木对接穗下胚轴木质部CTK和ABA流量的调节可能也是一种伴生现象; Y型嫁接2个不同接穗之间下胚轴解剖结构差异较大, 与二者之间CTK和ABA流量无显著差异的结果不符。因此, 棉花叶片早衰2种根-冠互作类型接穗下胚轴解剖结构与其木质部中CTK和ABA的流量无关。此外, 无论是地上部主导型还是根系主导型, 2个品种自身嫁接的接穗下胚轴结构差异在Y型嫁接与I型嫁接中不一致, 可能与Y型嫁接的2个接穗之间存在冠-冠互作有关。
Under potassium (K) deficiency, effect of root-shoot interaction on cotton leaf premature senescence includes shoot-dominated and root-dominated patterns. Indirect evidences from grafting study under K deficiency have suggested that scion hypocotyl of shoot-dominated pattern (CCRI41 and SCRC22 combination) may have the ability to synthesize, metabolize and/or radially transport cytokinin (CTK) and abscisic acid (ABA), while that of root-dominated pattern (CCRI41 and CCRI49 combination) mainly delivers CTK and ABA from roots. In the present study, we observed the anatomy of scion hypocotyls of both patterns under K deficiency (0.03 mmol L-1), and evaluated if it is associated with the flux of CTK and ABA in xylem. In terms of I type grafts (one scion grafted onto one rootstock) of shoot-dominated pattern, the anatomy of scion hypocotyl was clearly influenced by rootstocks, which is inconsistent with the fact that the delivery rate of CTK and ABA in scion xylem was mainly affected by shoot. For Y type grafts (two scions grafted onto one rootstock) of shoot-dominated pattern, the scion with greater CTK flux and lower ABA flux showed growth superiority as compared with its counterpart. However, the two processes referring to flux of phytohormone in scion xylem and scion growth likely occurred concomitantly but not causally. As the results of CTK and ABA flux in scion xylem, the anatomy of scion hypocotyls of root-dominated pattern was obviously altered by rootstock in I grafts. Nevertheless, they might be also concomitant processes rather than the relationship of cause and effect. Additionally, there were significant differences in anatomy between two different scions of Y grafts of root-dominated pattern, which was disagreement with the similar CTK and ABA flux in xylem of two scions. In conclusion, the scion hypocotyl anatomy of either shoot- or root-dominated pattern regarding cotton leaf premature senescence induced by K deficiency is independent of CTK and ABA flux in its xylem. In addition, the differences in scion hypocotyl anatomy between two cultivars in I graft type were inconsistent with those in Y graft type, which is possibly due to shoot-shoot interaction in Y graft.
嫁接是一种古老的植物无性繁殖技术, 广泛应用于果树生产, 最近几十年也用于蔬菜作物。此外, 嫁接也是研究植物根-冠互作和长距离信号传输的有效手段。将对缺钾敏感、衰老较快的棉花品种中棉所41 (CCRI41, 简称C41)分别与对缺钾敏感性低、衰老较慢的棉花品种鲁棉研22 (SCRC22, 简称S22)和中棉所49 (CCRI49, 简称C49)嫁接, 结果表明在缺钾诱导早衰的条件下根-冠互作对棉花叶片衰老的调节具有多样性, 可分为地上部主导型(C41与S22组合)[1, 2]和根系主导型(C41和C49组合)[3]。地上部主导型的叶片早衰(以光合速率和叶绿素含量表征)受接穗的影响比较大, 部分机制可能是因为接穗对其下胚轴木质部细胞分裂素(CTK、ZR+Z和iPA+iP)和脱落酸(ABA)的流量影响较大[2]。根系主导型的砧木则对叶片早衰和接穗下胚轴木质部CTK及ABA的流量影响比较大[3]。
对苹果[4]、桃[5]、甜樱桃[6]、葡萄[7]、柑橘[8]等果树的研究表明, 砧木控制接穗生长(树体大小)的机制之一是影响接穗木质部的输导能力, 如导管数量、直径和密度等。但就棉花叶片早衰而言, 无论是地上部主导型的接穗还是根系主导型的砧木, 对接穗下胚轴木质部CTK和ABA流量的影响与下胚轴木质部输导能力可能无直接联系, 因为它们对CTK和ABA流量的影响是相反的。如C41 (在地上部主导型组合中作为接穗, 在根系主导型组合中作为砧木)降低CTK流量但提高ABA流量, S22 (在地上部主导型组合中作为接穗)和C49 (在根系主导型组合中作为砧木)提高CTK流量但降低ABA流量[2, 3]。
在缺钾诱导早衰条件下, Wang等[2]对下胚轴木质部CTK和ABA流量的测定及杨志威[9]从砧木引入14C-ZR的研究均表明, 地上部主导型(C41和S22组合)的接穗下胚轴可能具有较强的CTK和ABA合成、代谢或横向运输能力; 根系主导型(C41和C49组合)的接穗下胚轴木质部则可能主要输导来自根系的CTK和ABA[3, 9]。已知CTK合成基因AtIPT3[10]、运输基因AtABCG14[11, 12]和ABA合成基因AtNCED3[13]、AtABA2[14]、AtAAO3[15]及运输基因AtABCG25[16]均主要在植物的维管系统中表达, 其中AtABCG14[11, 12]、AtABA2[14]和AtAAO3[15]可在下胚轴的维管系统中表达。此外, 棉花下胚轴韧皮部中(韧皮部+皮层)的CTK合成基因GhIPT (与AtIPT3同源性为55%)和运输基因GhPUP (与AtPUP2同源性为55%)表达量高于木质部, 而木质部中(木质部+髓)的ABA合成基因GhNCED (与AtNCED3同源性为66%)、活化基因GhBG (与AtBG1同源性为52%)和运输基因GhABCG (与AtABCG25同源性为64%)表达量高于韧皮部[17]。
本文以C41、S22和C49为材料, 分别进行单接穗单砧木(I型)和双接穗单砧木(Y型)嫁接, 在低钾(0.03 mmol L-1 K+)胁迫下诱导叶片早衰, 主要观察接穗和砧木下胚轴木质部和韧皮部的厚度、木质部管状分子、韧皮部筛管和伴胞、薄壁细胞及其活性(是否发生细胞壁次生加厚且木质化, 简称薄壁细胞硬化程度)和横向运输组分(木质部和韧皮部射线)等, 旨在探明棉花叶片早衰不同根-冠互作类型对下胚轴解剖结构的影响, 并判断接穗下胚轴的解剖结构与其木质部汁液中CTK和ABA的流量是否有关。
供试材料为C41、S22和C49, 种子由中国农业科学院棉花研究所和山东棉花研究中心提供。于中国农业大学光照培养室进行试验, 光照强度为500 μ mol cm-2 s-1, 光照/黑暗时间为14 h/10 h, 昼/夜温度为(28± 2)℃/(22± 2)℃, 相对湿度为70%~80%。种子经10%双氧水消毒15 min并清洗数遍后, 置去离子水中浸种24 h, 露白后播于不含K+的沙床, 出苗2 d后转移至K+浓度为0.1 mmol L-1的1/2改良Hoagland营养液中培养。
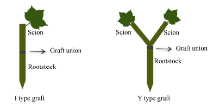
参照李博等[18]的方法, 对地上部主导型(C41和S22组合)和根系主导型(C41和C49组合)分别进行I型(接穗/砧木)和Y型(接穗+接穗/砧木)嫁接(图1)。I型嫁接采用劈接法, 用双面刀片将接穗切成楔形, 垂直插入砧木下胚轴长约1.0~1.5 cm的切口中, 然后用石蜡封口膜(Parafilm, USA)包裹固定。Y型嫁接是将2个接穗下部均切削成楔形, 等高并排插入砧木下胚轴切口内, 然后用封口膜层层缠紧。嫁接结束后将嫁接苗置刺有若干小孔的保鲜袋内(注意避免叶片与保鲜袋直接接触), 放在光强为80~100 μ mol cm-2 s-1、昼夜温度为29℃/20℃的条件下缓苗, 5 d后揭掉保鲜袋, 7 d后移至600 μ mol cm-2 s-1光强下开始低钾(0.03 mmol L-1 K+)胁迫。
嫁接苗成活后4周, 在距嫁接位点1.0 cm以外分别切取砧木和接穗0.5 cm长的下胚轴, 投入FAA固定液中, 真空泵抽气5 min。经系列乙醇脱水、二甲苯透明和石蜡包埋后, 利用RM2016石蜡切片机(徕卡仪器有限公司, 上海)横切包埋的下胚轴, 切片厚度10 μ m, 切片经粘片、脱蜡、番红-固绿对染、中性树胶封片等常规石蜡制片处理制作成永久制片, 采用配备DP72 CCD的Olympus BX51显微镜(Tokyo, Japan)观察和照相。
将制好的切片在20× 、50× 更好观察完整下胚轴结构, 根据标尺长度, 使用Image J软件Measure长度测量功能测量髓部、木质部、韧皮部、皮层+表皮厚度和下胚轴半径。
选择每处理3株代表性植株进行下胚轴制片, 在20× 下观察后每株选一张切片照相和测量, 利用SPSS Statistics 17.0.2 (IBM, 2009)的一般线性模型(general linear model procedure, GLM)对数据进行方差分析, 用Duncan’ s法进行多重比较。
无论是地上部主导型还是根系主导型, 无论是I型嫁接还是Y型嫁接, 缺钾诱导早衰条件下接穗对砧木下胚轴解剖结构的影响均很小(图片未给出)。
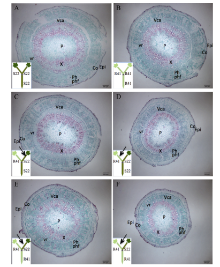
2.2.1 地上部主导型(C41和S22组合) 缺钾诱导早衰条件下, 2个品种自身嫁接接穗的下胚轴半径相似, 但下胚轴各部位厚度有差异, C41的髓部比较厚, S22的皮层+表皮比较厚, 二者木质部和韧皮部厚度相同(图2-A, D; 表1)。从图2还可看出, 与S22/S22相比, C41/C41接穗木质部靠近形成层的薄壁细胞数量多、早期形成的薄壁细胞硬化程度高、大口径管状分子多, 韧皮部差异不大, 但韧皮纤维量较多、韧皮纤维口径小、壁较厚, 维管射线(包括木射线和韧皮射线)整体口径较宽(图2-E, H)。
| 表1 棉花叶片早衰地上部主导型(中棉所41与鲁棉研22组合) I型嫁接接穗下胚轴各部分厚度及下胚轴半径 Table 1 Thickness of each tissue in cross section of scion hypocotyl and radius of scion hypocotyl in I type grafts (one scion grafted onto one rootstock, scion/rootstock) of shoot-dominated pattern (CCRI41 and SCRC22 combination) regarding cotton leaf premature senescence (μ m) |
C41/S22与C41/C41相比, 或S22/C41与S22/S22相比, 砧木改变后对接穗的髓部或皮层+表皮厚度有显著影响, 但对木质部和韧皮部厚度影响不大(图2和表1)。进一步观察木质部和韧皮部的组织结构, 发现与C41/C41相比, S22作为砧木使C41接穗下胚轴木质部早期形成的薄壁细胞硬化程度减轻、韧皮部有输导功能的组分(筛管+伴胞)增加、纤维细胞减少、韧皮纤维口径变小壁增厚(图2-G, H), 因此C41/S22的接穗下胚轴解剖结构总体上与S22/S22接穗更为相近(图2-A, C)。与S22/S22相比, C41作为砧木使S22接穗下胚轴木质部薄壁细胞的硬化程度明显增加、大口径射线增多、小口径管状分子减少、大口径管状分子增加, 使韧皮部有输导能力的部位变薄、有输导功能的韧皮部组份减少、韧皮纤维量增加(图2-E, F)。S22/C41的接穗下胚轴解剖结构总体上与C41/C41接穗相近(图2-B, D)。
2.2.2 根系主导型(C41和C49组合) 缺钾诱导早衰条件下, 2个品种自身嫁接接穗下胚轴差异较大, C49的皮层薄于C41、髓部小于C41, 但木质部和韧皮部均显著厚于C41, 且木质部的管状分子(尤其是大口径管状分子)和韧皮部中的韧皮纤维量明显多于C41 (图3-A, D)。此外, C49韧皮部的大口径射线少于C41。
C41/C49与C41/C41相比, 或C49/C41与C49/C49相比, 可看出砧木显著影响接穗下胚轴解剖结构(图3和表2)。C49作为砧木增加了C41接穗的木质部和韧皮部厚度, 但其髓部变小、皮层变薄(图3-C, D)。C41作为砧木显著减小了C49接穗下胚轴的木质部和韧皮部厚度, 对髓部影响不大, 皮层+表皮有所增厚(图3-A, B)。进一步观察木质部和韧皮部的组织结构, 发现与C41自身嫁接相比, C49作为砧木大幅增加了C41接穗下胚轴木质部的管状分子和薄壁细胞, 韧皮部中的韧皮纤维量也明显增多, 大口径射线量减少(图3-G, H)。C41/C49接穗下胚轴的解剖结构总体上与C49/C49接穗相似(图3-A, C)。与C49自身嫁接相比, C41作为砧木使C49接穗木质部管状分子和薄壁细胞大幅减少, 使下胚轴韧皮部大口径的射线增加、韧皮纤维减少(图3-E, F)。C49/C41接穗下胚轴的解剖结构与C41/C41接穗基本相同(图3-B, D)。
| 表2 棉花叶片早衰根系主导型(中棉所41与中棉所49组合)I型嫁接接穗下胚轴各部分厚度及下胚轴半径 Table 2 Thickness of each tissue in cross section of scion hypocotyl and radius of scion hypocotyl in I type grafts (one scion grafted onto one rootstock, scion/rootstock) of root-dominated pattern (CCRI41 and CCRI49 combination) regarding cotton leaf premature senescence (μ m) |
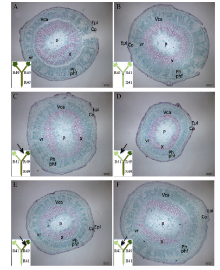
2.3.1 地上部主导型(C41和S22组合) 缺钾诱导早衰条件下, C41自身嫁接(C41+C41/C41)与S22自身嫁接(S22+S22/S22)相比, 接穗下胚轴半径相差不大, 但C41的韧皮部较厚、韧皮部射线较粗, S22木质部的管状分子则比较多(图4和表3)。此外, 无论砧木为何品种, 互相嫁接2个不同品种接穗的半径、木质部和韧皮部厚度、木质部管状分子均小于(少于)或显著小于(少于)自身嫁接(图4和表3), 提示Y型嫁接2个不同品种的接穗之间存在互相抑制作用。比较互相嫁接处理的2个接穗, 可见S22接穗下胚轴半径、木质部和韧皮部厚度(砧木为S22时除外)及木质部管状分子和薄壁细胞数量大于或多于C41接穗(图4-C~F; 表3), 且这种差异与砧木品种关系不大。
2.3.2 根系主导型(C41和C49组合) 缺钾诱导早衰条件下, C41自身嫁接(C41+C41/C41)与C49自身嫁接(C49+C49/C49)相比, 接穗下胚轴半径相差不大, 木质部和韧皮部厚度也基本相似, 但C41木质部靠近形成层的薄壁细胞多于C49、皮层较C49厚(图5和表4)。互相嫁接(C41+C49/C41、C41+C49/ C49)各接穗下胚轴木质部和韧皮部厚度均小于自身嫁接, C41+C49/C41的C49接穗和C41+C49/C49的C41接穗下胚轴半径与自身嫁接相比也显著降低, 提示互相嫁接的2个不同品种接穗之间存在互相抑制作用(图5和表4)。此外, 互相嫁接接穗下胚轴的皮层均较自身嫁接加厚, C41+C49/C49的C41接穗下胚轴韧皮部射线宽度小于C41自身嫁接(图5和表4)。比较互相嫁接处理的2个接穗, 可见与砧木品种相同的接穗相对于另一个接穗表现出明显的生长优势。如砧木为C41时, C41接穗下胚轴各部位的厚度和下胚轴半径大于C49接穗, 其中髓部厚度和下胚轴半径差异显著(图5-E, F); 砧木为C49时, C49接穗下胚轴各部位厚度和下胚轴半径大于C41接穗, 其中木质部和皮层+表皮的差异比较大(图5-C, D)。
| 表3 棉花叶片早衰地上部主导型(中棉所41与鲁棉研22组合)Y型嫁接接穗下胚轴各部分厚度及下胚轴半径 Table 3 Thickness of each tissue in cross section of scion hypocotyl and radius of scion hypocotyl in Y type grafts (two scions grafted onto one rootstock, scion+scion/rootstock) of shoot-dominated pattern (CCRI41 and SCRC22 combination) regarding cotton leaf premature senescence (μ m) |
| 表4 棉花叶片早衰根系主导型(中棉所41与中棉所49组合)Y型嫁接接穗下胚轴各部分厚度及下胚轴半径 Table 4 Thickness of each tissue in cross section of scion hypocotyl and radius of scion hypocotyl in Y type grafts (two scions grafted onto one rootstock, scion+scion/rootstock) of root-dominated pattern (CCRI41 and CCRI49 combination) regarding cotton leaf premature senescence (μ m) |
本试验I型嫁接结果表明, 在低钾胁迫诱导早衰的条件下, 棉花叶片早衰根系主导型的2个品种(C41和C49)接穗下胚轴结构差异很大, 地上部主导型的2个品种(C41和S22)差异相对较小, 但2种类型的砧木均可明显改变接穗的下胚轴结构, 使其与砧木品种更为接近(图2和图3)。
低钾胁迫条件下, 地上部主导型I型嫁接的砧木对接穗下胚轴木质部CTK和ABA的流量影响较小[2], 因此该类型下胚轴解剖结构与其木质部的植物激素流量可能受到不同机制的调节。地上部主导型的Y型互相嫁接, 无论砧木为何品种, S22接穗下胚轴木质部汁液中的CTK流量在低钾胁迫下显著高于C41接穗, ABA流量则显著低于C41接穗[2]。本研究S22接穗较C41接穗下胚轴在缺钾胁迫下的生长优势虽然与砧木的关系也不大, 但推测S22接穗在低钾胁迫下的抗早衰能力(指下胚轴木质部的高CTK流量和低ABA流量)与下胚轴生长优势是一种伴生关系而非因果关系。因为S22接穗的木质部厚度和薄壁细胞数量明显大于(多于) C41接穗, 考虑到棉花下胚轴木质部薄壁细胞可能是ABA生物合成的场所[13, 15, 17], 所以推测S22的ABA流量可能高于C41, 但实际情况相反。叶片早衰根系主导型I型嫁接的接穗下胚轴解剖结构(图3)与其木质部的植物激素流量[3]在低钾胁迫下虽然均受到砧木的显著影响, 但这2个过程可能也是一种伴生关系而不是因果关系。因为C49作为砧木显著增加了C41接穗下胚轴的木质部厚度及薄壁细胞(潜在的ABA合成部位)数量, 所以有可能提高ABA的流量, 但实际上C49作为砧木降低了C41接穗木质部的ABA流量[3]。根系主导型Y型互相嫁接的C41和C49接穗下胚轴解剖结构在低钾胁迫下差异较大(图5), 但二者木质部的CTK和ABA流量无显著差异[3], 说明下胚轴解剖结构与其运输植物激素的能力是2个独立的过程。
双子叶植物下胚轴的生长和解剖结构主要与形成层的活性和分化能力有关。已知生长素(auxin)、CTK、赤霉素(GA)和油菜素内酯(BRs)协同作用维持形成层的活性[19, 20], 木质部的分化和生长发育主要受GA[21, 22, 23, 24]和BRs[25, 26, 27]的调节。Ragni等[23]利用突变体及嫁接技术研究发现, GA信号途径(DELLA)对木质部扩张的影响不能通过嫁接传递, 而GA本身可作为移动信号调节木质部发育, 野生型(Ler)无论作为接穗还是砧木均能恢复GA合成突变体ga1-3下胚轴木质部的扩张能力。
本研究表明, 棉花叶片早衰2种根-冠互作类型的砧木均可明显改变接穗下胚轴解剖结构, 但接穗对砧木下胚轴解剖结构的影响很小, 说明接穗下胚轴的生长和发育受到了来自砧木的信号影响, 但砧木下胚轴的生长和发育表现出更多的自治性。这其中涉及的长距离和/或局部信号及其调节机制可能比较复杂, 需要开展系统和深入的研究。
关于棉花叶片早衰和木质部CTK及ABA流量的研究未发现Y型嫁接具有明显和一致的冠-冠互作[2, 3], 但本文缺钾胁迫条件下Y型嫁接接穗下胚轴解剖结构的结果可能需要用冠-冠互作来解释。
无论是地上部主导型还是根系主导型, 2个品种自身嫁接的接穗下胚轴结构差异在Y型嫁接与I型嫁接中不一致。地上部主导型2个品种(C41和S22) Y型自身嫁接接穗下胚轴的半径虽然也像I型自身嫁接一样无明显差别, 但组织水平的差异不一致。如C41的I型自身嫁接木质部管状分子多于S22 (图2), 但Y型自身嫁接的木质部管状分子少于S22 (图4)。又如C41和S22的I型嫁接接穗韧皮部厚度和射线口径无明显差别(图2), 但Y型嫁接C41的韧皮部较厚, 韧皮部射线的口径较大(图4)。根系主导型的I型自身嫁接, C49接穗的半径、木质部和韧皮部厚度均显著且大幅高于C41接穗(图3), 但2个品种Y型自身嫁接接穗的上述几个指标无明显差异(图5)。除此之外, 地上部主导型和根系主导型Y型互相嫁接的2个不同品种接穗均表现出互相抑制作用(与自身嫁接相比), 其中地上部主导型接穗之间的互相抑制与砧木关系不大(图4), 而根系主导型接穗之间的互相抑制与砧木有关(图5)。
在缺钾诱导棉花叶片早衰的条件下, 无论是根-冠互作的地上部主导型组合还是根系主导型组合, 无论是I型嫁接还是Y型嫁接, 砧木对接穗下胚轴的解剖结构影响较大; Y型嫁接接穗下胚轴的解剖结构还受到冠-冠互作的影响。接穗下胚轴解剖结构不能解释其木质部CTK和ABA的流量。未来需要开展分子生物学和组织化学等方面的研究, 为棉花叶片早衰的地上部主导型接穗下胚轴合成、代谢和/或横向运输CTK、ABA的能力提供直接证据, 并明确根系主导型接穗下胚轴是否主要行使输导来自根系的CTK和ABA的功能。此外, 还需要研究Y型嫁接冠-冠互作影响接穗下胚轴解剖结构的信号物质和调节机制, 及冠-冠互作与根-冠互作之间的关系。
致谢: 感谢中国农业大学生物学院王幼群副教授在制片技术上提供的帮助, 感谢中国农业大学作物化控研究中心王崧嫚同学在图片整理过程中提供的帮助。
The authors have declared that no competing interests exist.
作者已声明无竞争性利益关系。
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|