田间种植可溶性糖含量不同的3个菜用大豆品种(系), 在R5.5、R6、R6.2、R6.5和R7期取样, 分析籽粒种皮、子叶和胚轴中蔗糖含量及4种关键酶活性动态, 结果表明, 籽粒不同部位蔗糖积累呈先增加后下降的趋势, R6.2期是高峰期, 此时期品种台292、中科毛豆1号和品系121的胚轴蔗糖含量比子叶分别高57.6%、53.6%和44.2%; 比种皮分别高71.6%、75.3%和73.6%。由于子叶干重占整粒重90%以上, 因此整个籽粒的蔗糖含量主要由子叶决定。子叶的蔗糖磷酸合酶(SPS)活性高于胚轴和种皮, 在R6.2期表现更加明显, 且蔗糖含量高的品系121子叶中SPS活性高于另外2个品种; 蔗糖合酶(SS)在籽粒形成期活性变化呈前期高于后期的趋势, 最高值出现在灌浆前期R5.5期胚轴中; 两种转化酶活性变化差异较大, 中性转化酶(NI)活性一直呈不断下降趋势; 籽粒不同部位NI活性无明显差异, 而酸性转化酶(AI)活性差异较大; 胚轴和子叶中AI活性明显低于种皮, 且种皮中AI活性与种皮中蔗糖积累显著负相关(
Three vegetable soybean varieties (lines) with different soluble sugar contents were grown in field condition. Sucrose contents and four critical enzymes activities in different parts of seed were analyzed at R5.5, R6, R6.2, R6.5, and R7 stages, respectively. Sucrose contents of different seed parts increased at early seed filling stage and then decreased at late stage with the peak value at R6.2 stage. Sucrose content in hypocotyl was 57.6%, 53.6%, 44.2% greater than that of cotyledon, and 71.6%, 75.3%, 73.6% greater than that of seed coat at R6.2 stage for the three varieties (lines), respectively. The cotyledon contributed 90% dry weight to the total seed dry weight, and thus the sucrose content of the whole seed was primarily determined by sucrose content in cotyledon. Cotyledon had the highest activity of sucrose phosphate synthase (SPS). The cotyledon SPS activity of cv. 121 was much higher than those of the other two varieties (lines). The sucrose synthase (SS) activity of different seed parts was higher in early seed filling stage than in late filling stage. The highest value of SS activity was observed in hypocotyl at R5.5 stage. Greater differences were found between acid invertase (AI) and neutral invertase (NI) activities. NI activity was higher in early seed filling stage and then decreased till R7 stage. Significant differences were observed for AI activities among three seed parts at the same seed filling stage but there were no differences for NI activities. AI activity in seed coat was much higher than those in hypocotyl and cotyledon. Negative relationship was found between AI activity and sucrose content in seed coat (
菜用大豆是一种特用大豆(Glycine max L. Merr.), 是指在R6 (鼓粒盛期)至R7 (初熟期)生育期间采青食用的大豆。鲜食期的菜用大豆籽粒富含多种营养元素, 有较高的食用品质, 越来越受到消费者的欢迎[1-4]。在众多的选择标准中, 食用品质是人们选择食品时最主要的方面[5]。果蔬食用品质是由人类味觉所能辨识的酸、甜、苦和咸4个基本味道决定的[6], Jouquand等[7]、Masuda[8]和Shanmugasundaram等[9]认为糖分和一些挥发类物质是影响果蔬食用品质的最主要化学成分, 其中, 籽粒糖分含量对菜用大豆的食用品质好坏影响显著。而蔗糖是菜用大豆采摘期含量最多的可溶性糖, 可占可溶性糖总量的71%左右, 其含量的高低直接影响菜用大豆的食用品质和消费者的喜爱程度[9-10]。
蔗糖是植物光合作用的初产物, 通过韧皮部以源库两端的渗透压为驱动力运输到库端, 在相关酶的催化下分解、重新合成和代谢[11]。研究发现, 植物体内的蔗糖代谢主要由蔗糖磷酸合酶(sucrose phosphate synthase, SPS)、蔗糖合酶(sucrose synthase, SS)、酸性转化酶(acid invertase, AI)和中性转化酶(neutral invertase, NI)调节[12]。SPS主要分布在植物的叶肉细胞中, 在不同的作物中均发现与蔗糖的积累呈正相关[13,14,15]。Ishimaru等[16]将玉米 SPS 基因转入马铃薯后, 叶片中蔗糖磷酸合酶活性提高200%, 同时块茎中的蔗糖含量也显著增加。SS既可以催化蔗糖的合成也可以催化蔗糖的分解, 对植物体内蔗糖的代谢起着至关重要的作用。蔗糖分解后的产物UDPG是淀粉合成的前体物质, 马铃薯、番茄和小麦中均发现淀粉的积累强度与SS活性的增加密切相关[17,18,19]。通过对不同基因型大豆叶片中SS活性的测定发现, SS对蔗糖的分解在成熟度不同的叶片中具有差异, 且均远大于合成作用[20]。AI和NI被统称为转化酶(invertase, Inv), 同时也有关于碱性转化酶(alkaline invertase)的报道, 但一般认为碱性转化酶和中性转化酶是同一种酶[21,22]。通常, 在植物的幼嫩组织和器官中转化酶的活性较高, 随着组织和器官的成熟, 转化酶的活力逐渐下降, 很多研究者推断转化酶与植物的生长和器官的建成关系密切[23,24]。水稻籽粒中的AI活性在籽粒灌浆前期活性较灌浆后期高, 与SS一起通过对蔗糖的降解来调控籽粒生长[25]。但不同部位结合的转化酶的作用有所差异, 同液泡结合型转化酶相比, 细胞壁结合型转化酶活性高且与籽粒中的淀粉积累呈显著正相关[26]。孙庆泉等[27]发现, 玉米籽粒中的蔗糖转化酶活性与籽粒充实有关, 较高的转化酶活性有利于玉米的高产。但目前国内关于菜用大豆籽粒中蔗糖代谢相关酶活性的研究还未见报道。本文报道了菜用大豆籽粒中不同组织蔗糖在籽粒形成期的变化动态, 解析了关键酶的活性变化及其与蔗糖积累的关系, 对揭示蔗糖积累机制, 改善菜用大豆品质, 培育蔗糖含量高的品种具有重要理论意义。
试验于2012年在中国科学院东北地理与农业生态研究所哈尔滨试验场内进行。土壤为典型的黑土, pH 6.6, 含有机质29.1 mg kg-1、全氮2.3 mg kg-1、全磷1.3 mg kg-1、全钾18.9 mg kg-1、碱解氮167.3 mg kg-1、速效磷21.8 mg kg-1、速效钾194.9 mg kg-1。供试品种为可溶性糖含量不同的3个菜用大豆品种(系), 其中台292为有限结荚型, 中科毛豆1号和品系121为亚有限结荚型; 台292为紫花, 中科毛豆1号和品系121为白花(表1); 试验采取小区随机区组, 3次重复。小区为5垄5 m行长, 行距70 cm, 株距5 cm。播种日期为2012年5月3日, 开花时对植株挂牌, 参考MinKuo等[28]定义, 分别在R5.5 (鲜籽粒扁且未充满整个豆荚), R6 (鲜籽粒充满整个豆荚), R6.2 (鲜籽粒充满整个豆荚且饱满), R6.5 (鲜籽粒胚轴组织稍有变黄), R7 (整个籽粒颜色变黄)随机取样。每小区取10株, 取植株中上部豆荚, 将鲜籽粒以手术刀分为种皮、子叶和胚轴, 称重后测定蔗糖含量和相关酶活性。鉴于酶活性普遍存在日变化差异, 每次取样时间均固定在上午9:00至10:00。
| 表1 供试菜用大豆品种(系)农艺特性 Table 1 Agronomic characteristics of different vegetable soybean varieties (lines) |
将鲜籽粒不同部位样品放入鼓风干燥器(永光明-101)中105℃杀青30 min, 80℃烘至恒重, 再用粉碎器(IKA- WERKE)粉碎, 过147 μm筛。称取0.4 g过筛干样放入 10 mL离心管中, 加80%乙醇4 mL后于80℃水浴锅中提取, 每10 min用涡漩仪混匀一次, 提取40 min后4500× g 离心3 min, 取上清液转移到15 mL离心管中, 重复提取3次, 合并上清液后用真空旋转蒸干仪(Eppendoft-5305)浓缩至1 mL, 过0.2 μm滤膜后用HPLC法测定蔗糖含量。检测器为Waters2414示差折光检测器(RID), 色谱柱为BENSON Polymeric 1000-0 BP-100 Ca2+ Carbohydrate Column (250 mm×4 mm i.d.), 流量为0.5 mL min-1, 蔗糖标准品为Sigma公司色谱纯级, 实验用水为超纯水。
1.3.1 酶液的提取 称取鲜籽粒不同部位0.5 g, 用4倍体积的100 mmol Tris-HCl (pH 7.0)缓冲液(内含10 mmol L-1 MgCl2、2 mmol L-1 EDTA、2%乙二醇、20 mmol L-1巯基乙醇)10 mL冰浴中快速研成匀浆, 以单层尼龙布过滤, 将滤液于4℃ 9 500× g 离心30 min, 取1 mL上清液过Sephadex G-25 (5 mL)柱除去小分子物质, 收集洗脱液测定酶活力。
1.3.2 SS活力测定 取0.3 mL的反应混合液, 内含 50 mmol L-1 Tris-HCl pH 7.0、10 mmol L-1 MgCl2、2 mmol L-1EDTA、2%乙二醇、20 mmol L-1巯基乙醇、100 mmol L-1的蔗糖、50 mmol L-1尿苷二磷酸葡萄糖(UDPG), 另加入0.2 mL酶液在37℃水浴中反应20 min, 在沸水浴保温 4 min, 流水冷却, 测定体系中蔗糖含量。
1.3.3 SPS活力测定 取0.3 mL的反应混合液, 内含50 mmol L-1Tris-HCl pH 7.0、10 mmol L-1 MgCl2、2 mmol L-1 EDTA、2%乙二醇、20 mmol L-1巯基乙醇、10 mmol L-1 6-磷酸果糖(F6P)、3 mmol L-1 UDPG, 另加入0.2 mL酶液在30℃水浴中反应20 min, 在沸水浴保温4 min, 流水冷却, 测定体系中蔗糖含量。
1.3.4 AI (酸性)活力测定 取0.30 mL的反应混合液, 含50 mmol L-1 Tris-HCl pH 4.5、10 mmol L-1 MgCl2、 2 mmol L-1 EDTA、 2%乙二醇、20 mmol L-1巯基乙醇、 100 mmol L-1的蔗糖, 另加入0.2 mL酶液, 37℃反应30 min, 在沸水浴保温4 min, 流水冷却, 测定体系中蔗糖含量。
1.3.5 NI(中性)活力测定 测定方法与酸性转化酶类似, 缓冲液pH调为7.2。
用Microsoft Excel 2010和SPSS 13.0软件进行数据统计分析, SigmaPlot 10.0作图。
虽然3个菜用大豆品种(系)籽粒大小不同, 但籽粒干重的变化趋势一致(表2)。从鼓粒期开始菜用大豆籽粒的干物重呈快速增加的趋势, R6.5期达到最高, R7时期略有下降。在菜用大豆籽粒的成熟阶段, 子叶始终是其干物重最大的部分, 其次为种皮和胚轴。在鼓粒始期R5.5期, 品种台292、中科毛豆1号和品系121的子叶干物重分别占籽粒干物质重的74.6%、75.7%和77.5%, 而所占比例最高时期为R7期, 分别为92.0%、91.4%和89.9%。菜用大豆种皮干物重则随成熟期不断下降。在R5.5期, 台292、中科毛豆1号和品系121的籽粒种皮分别占籽粒干物质重的22.0%、21.2%和19.6%, 到R7期则分别下降到5.6%、6.4%和7.7%。菜用大豆胚轴干物重占整个籽粒的干物重比例非常小并且变幅也较小, 其中, 台292为2.1%~3.4%、中科毛豆1号为2.1%~3.1%、品系121为2.2%~2.9%。
| 表2 菜用大豆台292、中科毛豆1号和品系121籽粒不同部位干重变化 Table 2 Dry weight changes of different parts of seed during seed filling in three vegetable soybean varieties (lines) (mg) |
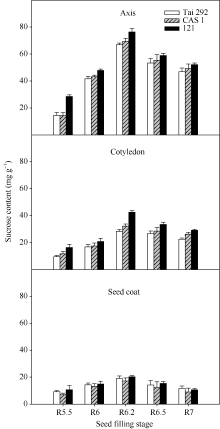
菜用大豆籽粒中胚轴的蔗糖含量最高, 种皮的蔗糖含量最低(图1)。虽然3个品种的蔗糖含量有所差异, 但在籽粒成熟过程中的积累趋势一致, 籽粒各部位均表现为鼓粒前期较低, 随着籽粒的膨大不断增高, 在R6.2期达最高值后又缓慢下降直到籽粒成熟。在蔗糖含量最低的R5.5期, 台292、中科毛豆1号和品系121籽粒胚轴中蔗糖相对
含量分别为14.5、14.3和28.52 mg g-1, 比子叶中分别高34.5%、18.5%和42.7%, 比种皮中分别高37.2%、51.3%和62.5%。在蔗糖含量最高的R6.2期, 台292、中科毛豆1号和品系121的胚轴蔗糖相对含量分别为66.9、69.4和76.4 mg g-1, 比子叶中分别高57.6%、53.6%和44.2%; 比种皮中蔗糖含量分别高出71.6%、75.3%和73.6%。
图2表明, 3个品种(系)种皮中SPS活性峰值出现在R6期, 随后迅速下降, 在籽粒形成的中后期维持在较低水平。子叶中的SPS活性表现为单峰曲线变化, 峰值出现在R6.2期, 随后缓慢下降直至籽粒成熟。在籽粒的形成的中后期, 子叶中SPS活性明显高于其他部位。在R6.2期, 台292、中科毛豆1号和品系121子叶中SPS活性比种皮中分别高72.0%、59.2%和58.6%; 比胚轴中分别高27.3%、26.9%和13.4%; 在R6.5期, 台292、中科毛豆1号和品系121子叶中SPS活性比种皮中分别高41.9%、50.2%和66.2%; 比胚轴中分别高24.0%、20.3%和39.4%。同时, 不同品种间子叶SPS活性差异明显, 在R6.2期品系121的子叶SPS活性比台292和中科毛豆1号分别高3.2%和13.4%, 而在R6.5期则分别高21.2%和12.5%。台292和中科毛豆1号胚轴SPS活性变化表现为前期明显上升, 中后期下降不明显, 而品系121胚轴SPS活性则表现为前期上升不明显, R6.2期以后迅速下降。
菜用大豆籽粒成熟过程中, 籽粒不同部位SS活性一般初期最高, 随后便缓慢降低, 在灌浆中期有所上升, 然后再一次降低直到籽粒成熟(图2)。籽粒不同部位SS活性有所差异, 胚轴明显高于子叶和种皮。在活性较高的R5.5期, 台292、中科毛豆1号和品系121胚轴中SS活性分别为0.37、0.42和0.45 μmol g-1 min-1干重, 比种皮分别高21.4%、24.8%和25.5%; 比子叶分别高32.1%、13.7%和16.6%。籽粒灌浆的R6.2期为台292和中科毛豆1号SS活性的低值期, 却是品系121的高值期, 胚轴中SS活性分别为0.27、0.28和 0.35 μmol g-1 min-1干重, 比种皮分别高出55.8%、16.3%和37.6%; 比子叶分别高46.6%、45.2%和47.8%。
籽粒灌浆过程中菜用大豆籽粒不同部位2种转化酶活性变化差异较大(图3)。子叶和胚轴中的AI活性在籽粒形成期差异不明显, 同种皮中AI相比, 始终维持在相对较低水平。种皮的AI活性在灌浆初期活性最高, 随后一直处于不断下降状态, 在R6.2期达到最低, 随后又有小幅度上升。在活性最高的R5.5期, 台292、中科毛豆1号和品系121种皮中的AI活性分别为0.32、0.30和0.21 μmol g-1 min-1干重, 比子叶中分别高71.1%、70.0%和61.6%; 比胚轴中分别高73.9%、69.1%和62.7%。而在活性最低的R6.2期, 台292、中科毛豆1号和品系121种皮中的AI活性分别为0.14、0.11和0.11 μmol g-1 min-1干重, 比子叶中分别高45.5%、38.5%和27.9%; 比胚轴中分别高50.5%、32.8%和18.1%。
籽粒不同部位的NI活性差异较小, 且在籽粒形成期变化相似(图3)。在籽粒灌浆前期R5.5和R6期活性高, 随后便逐渐下降直到籽粒成熟。同R5.5期相比, R6.2期的台292、中科毛豆1号和品系121NI活性在种皮中分别降低48.5%、51.8%和51.9%; 子叶中分别降低18.1%、43.0%和49.0%; 胚轴中分别降低62.6%、39.3%和43.5%。同R5.5期相比, R7期的台292、中科毛豆1号和品系121NI活性在种皮中分别降低63.8%、63.2%和48.1%; 子叶中分别降低62.2%、59.6%和57.1%; 胚轴中分别降低65.9%、62.5%和45%。
表3表明, 在种皮中只有AI ( r = -0.59*)活性与蔗糖的积累显著相关, 而子叶中SS ( r = -0.56*)、AI ( r = -0.63*)和NI ( r = -0.76**) 3种酶活性与蔗糖的积累呈显著负相关; SPS ( r = 0.88*)活性与蔗糖的积累呈显著正相关。胚轴中SS ( r = -0.57*)和NI ( r = -0.72**)活性与蔗糖的积累呈显著负相关; SPS ( r = 0.60*)与蔗糖的积累呈显著正相关。进一步分析发现, 用与蔗糖积累正相关的酶活性(SPS)和与蔗糖积累负相关的酶活性(SS+AI+NI)相减所得净酶活性与籽粒中蔗糖积累呈显著正相关( r = 0.53**)
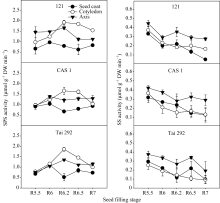 | 图2 籽粒形成期菜用大豆籽粒不同部位SPS和SS活性变化Fig. 2 Changes of SPS and SS activities in different parts of seed during seed filling period in vegetable soybean |
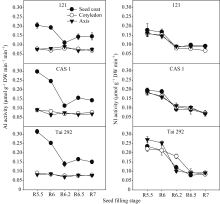 | 图3 籽粒形成期菜用大豆籽粒不同部位AI和NI活性变化Fig. 3 Changes of AI and NI activities in different parts of seed during seed filling period in vegetable soybean |
| 表3 菜用大豆籽粒不同部位蔗糖含量与不同酶活性的相关系数 Table 3 Correlation coefficients between sucrose content and enzyme activities in different parts of seed of vegetable soybean |
可溶性糖含量的多少直接关系菜用大豆的口感, 从而影响一个品种在市场中受欢迎的程度。蔗糖作为菜用大豆鲜食期含量最多的可溶性糖, 是菜用大豆栽培和品种选育研究的焦点。籽粒是光合产物的主要贮存地点, 蔗糖在籽粒中的合成与分解不仅关系着菜用大豆的品质, 还参与源库之间的调节从而影响产量[8,29]。本研究发现, 菜用大豆籽粒不同部位的蔗糖含量差异显著, 为胚轴>子叶>种皮。由于子叶约占整个籽粒干重的90%以上, 是籽粒的最大组成部分, 所以子叶的绝对蔗糖含量决定了整个籽粒的蔗糖含量。已有研究表明, 胚(子叶+胚轴)作为蔗糖最主要的贮存场所, 在籽粒灌浆过程中经多种酶催化的代谢反应为籽粒生长提供所需原料和能量[28]。本研究表明子叶是菜用大豆籽粒蔗糖代谢的主要场所, 但只有SPS活性与子叶和胚轴的蔗糖含量显著正相关, 并且SPS活性的高峰期(R6.2)也是各部位蔗糖积累的高峰期(R6.2), 而R6.2期正处于菜用大豆鲜荚的采摘期[30], 较高的SPS酶活性有利于菜用大豆食用品质的提高, 说明籽粒中蔗糖的积累是在SPS催化下完成的。SS是催化蔗糖降解与蔗糖合成的可逆酶[31]。本研究发现, 蔗糖浓度较高的胚轴中SS活性明显高于其他部位, 这是因为较高浓度的蔗糖分子会抑制SS的合成作用, 而促进SS对蔗糖的分解作用[32]。本研究同时发现, 籽粒不同部位SS活性均与蔗糖的积累呈负相关, 表明菜用大豆籽粒中SS的主要作用是对蔗糖的分解。虽然SS的存在不利于蔗糖的积累, 但是对光合产物的积累和产量的形成却具有重要意义。子实型作物的产量依赖于源端对籽粒光合产物的不断供应, 蔗糖是光合产物在源库之间主要运输形式, 源库之间蔗糖浓度的梯度差将调节籽粒中蔗糖的输入速率, 而籽粒中蔗糖的浓度正是在这些关键酶共同调节下完成的[29,33,34]。AI调节蔗糖在库端的卸载和转运已经在很多植物中证实[35], 但本研究发现籽粒灌浆过程中不同部位AI活性变化较大, 只有种皮中AI活性变化与种皮蔗糖积累的相关性达到显著水平且活性显著高于其他部位, 但未引起相同时期种皮蔗糖含量变化, 所以推断种皮是AI发挥调节蔗糖在库端的卸载和转运功能的主要场所, 这与种皮高度维管化有关[36]。刘慧英和朱祝军[37]报道NI的活性在未成熟的果实中较高, 会随着果实的成熟逐渐降低。本研究也发现菜用大豆籽粒不同组织的NI活性差异较小, 且变化趋势相似, 在灌浆初期活性高并随着籽粒的成熟呈一直下降趋势。由此认为NI的主要功能是调节植物体内己糖的积累水平间接调控蔗糖的代谢[38,39]。本研究发现, 用蔗糖积累正相关的酶活性(SPS)和蔗糖积累负相关的酶活性(SS+AI+NI)相减所得净酶活性与籽粒中蔗糖积累呈显著正相关( r = 0.53**), 说明不是某种酶活性的绝对增加或减少决定着籽粒蔗糖的含量。综合推断, 当种皮中AI活性不高时, 源端输入的蔗糖不能及时分解而转运到籽粒中, 会导致SPS催化的底物缺失而降低活性, 致使籽粒中蔗糖含量下降; 在SS活性较高时, 籽粒中蔗糖分解速度加快, 虽然不利于蔗糖的积累却加大了源库两端蔗糖的梯度差, 从而促进蔗糖向籽粒的运输。此外, 本研究菜用大豆品系121的蔗糖含量较高, 其籽粒中4种关键酶活性一般也高于另外2个品种, 说明不同品种相关酶基因的表达存在明显差异。因此在选育品种时应注意, 蔗糖代谢关键酶活性在合成和分解两个方向上均较高的品系更有潜力发展成高蔗糖含量品种, 这方面的工作有待深入探讨。
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|